


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 19-7-2017
Date: 28-9-2018
Date: 28-9-2018
|
Example of First order Reactions
Some common reactions which follow first order kinetics are listed below :
(1) Decomposition of N2O5 in CCl4 solution. Nitrogen pentoxide in carbon tetrachloride solution decomposes to form oxygen gas,
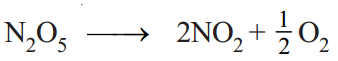
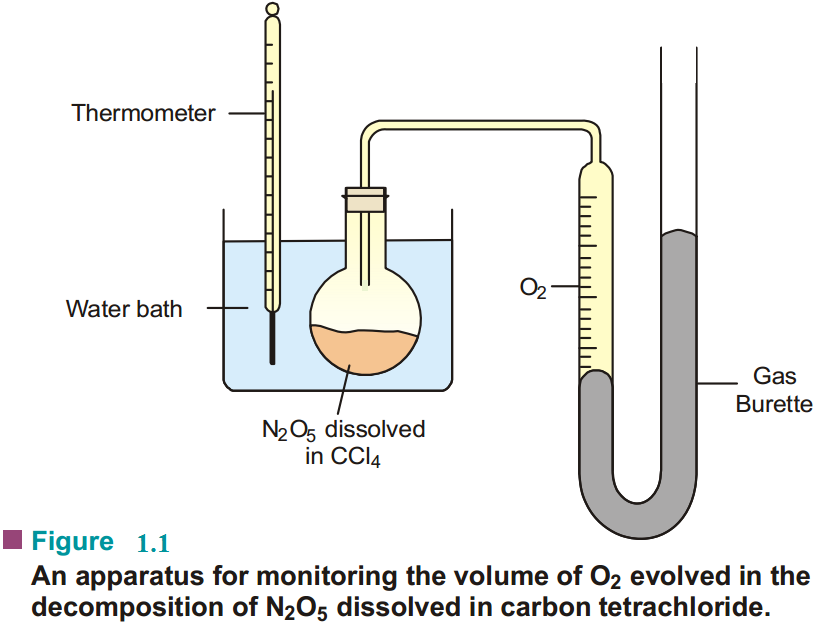
The reaction is carried in an apparatus shown in Fig. 1.1. The progress of the reaction is monitored by measuring the volume of oxygen evolved from time to time.
If Vt be the volume of O2 at any time t and V∞ the final volume of oxygen when the reaction is completed, the V∞ is a measure of the initial concentration of N2O5 and (V∞– Vt) is a measure of undecomposed N2O5 (a–x) remaining at time t. Thus,

On substituting values of V∞, (V∞– Vt) at different time intervals, t, the value of k is found to be constant. Thus it is a reaction of the first order.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|