


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-9-2018
Date: 30-9-2020
Date: 23-9-2020
|
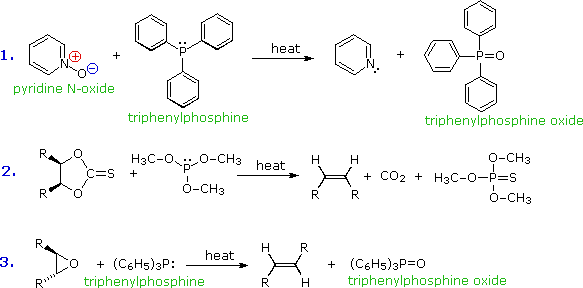
Trivalent phosphorus is easily oxidized. In contrast with ammonia and amines, phosphine and its mono and dialkyl derivatives are pyrophoric, bursting into flame on contact with the oxygen in air. The affinity of trivalent phosphorus for oxygen (and sulfur) has been put to use in many reaction systems, three of which are shown here. The triphenylphosphine oxide produced in reactions 1 & 3 is a very stable polar compound, and in most cases it is easily removed from the other products. Reaction 2 is a general formulation of the useful Corey-Winter procedure for converting vicinal glycols to alkenes.
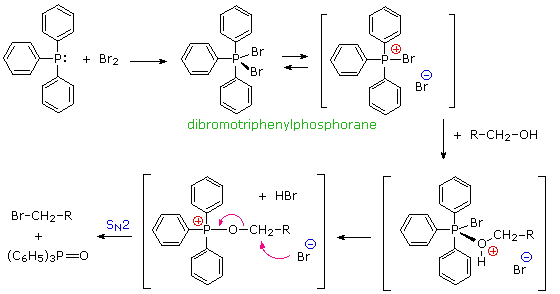
Triphenylphosphine is also oxidized by halogens, and with bromine yields dibromotriphenylphosphorane, a crystalline salt-like compound, useful for converting alcohols to alkyl bromides. As in a number of earlier examples, the formation of triphenylphosphine oxide in the irreversible SN2 step provides a thermodynamic driving force for the reaction.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|