


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-7-2016
Date: 28-1-2022
Date: 1-7-2019
|
When considering compounds having two or more double bonds in a molecule, it is useful to identify three distinct ways in which these functions may be oriented with respect to each other. First, the double bonds may be separated by one or more sp3-hybridized carbon atoms, as in 1,5-hexadiene. In this circumstance each double bond behaves independently of the other, and we refer to them as isolated. A second relationship has the double bonds connected to each other by a single bond, as in 1,3-hexadiene, and we refer to this arrangement as conjugated. Finally, two double bonds might share a carbon atom, as in 1,2-hexadiene. The central carbon atom in such a system is sp-hybridized, and we call such double bonds cumulated. These three isomers are shown in the following diagram, and three other similar isomers will be displayed on clicking the Change Examples button. In cases where stereoisomers are possible only the E-isomer is shown.
Another stereoisomeric factor associated with conjugated dienes will be demonstrated by clicking the Change Examples button a second time. Rotation about the single bond joining the two double bonds (colored blue) converts a trans-like s-trans conformation to its s-cis form. The energy barrier to this conformational isomerization is normally low, and the s-trans conformer is often more stable than the s-cis conformer, as shown in the diagram.
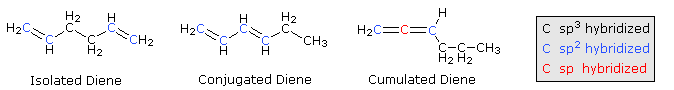
These categories are based on more than obvious structural variations. We find significant differences in the chemical properties of dienes depending on their structural type.
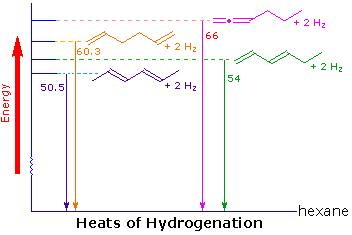
For example, catalytic hydrogenation converts all the dienes shown here to the alkane hexane, but the heats of reaction (heat of hydrogenation) reflect characteristic differences in their thermodynamic stability. This is illustrated in the diagram on the right. Taking the heat of hydrogenation of 1-hexene (30.1 kcal/mole) as a reference, we find that the isolated diene, 1,5-hexadiene, as expected, generates double this heat of reaction on conversion to hexane. The cumulated diene, 1,2-hexadiene, has a 6 kcal/mole higher heat of reaction, indicating it is less stable than the isolated diene by this magnitude. On the other hand, conjugation of double bonds seems to stabilize a diene by about 5 kcal/mole. The increase in stability of 2,4-hexadiene over 1,3-hexadiene (both are conjugated) is due to the increased double bond substitution of the former, a factor noted earlier for simple alkenes.
The stabilization of dienes by conjugation is less dramatic than the aromatic stabilization of benzene. Nevertheless, similar resonance and molecular orbital descriptions of conjugation may be written. A resonance description, such as the one shown here, involves charge separation, implying a relatively small degree of stabilization.
CH2=CH-CH=CH2  (+)CH2-CH=CH-CH2:(–)
(+)CH2-CH=CH-CH2:(–)
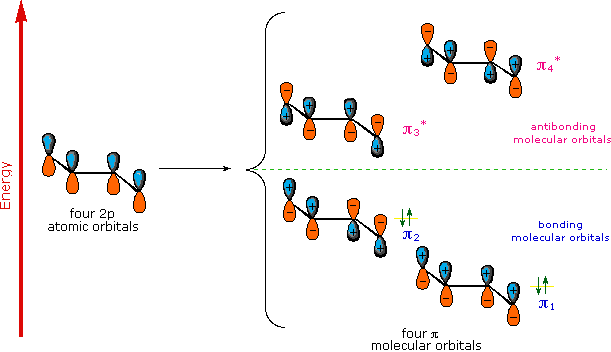
A molecular orbital model for 1,3-butadiene is shown below. Note that the lobes of the four p-orbital components in each pi-orbital are colored differently and carry a plus or minus sign. This distinction refers to different phases, defined by the mathematical wave equations for such orbitals. Regions in which adjacent orbital lobes undergo a phase change are called nodes. Orbital electron density is zero in such regions. Thus a single p-orbital has a node at the nucleus, and all the pi-orbitals shown here have a nodal plane that is defined by the atoms of the diene. This is the only nodal surface in the lowest energy pi-orbital, π1. Higher energy pi-orbitals have an increasing number of nodes.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|