


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-9-2020
Date: 11-9-2018
Date: 11-9-2018
|
An electrocyclic reaction is the concerted cyclization of a conjugated π-electron system by converting one π-bond to a ring forming σ-bond. The reverse reaction may be called electrocyclic ring opening.
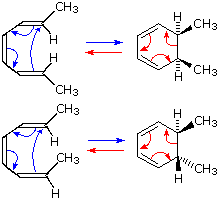

Two examples are shown on the right. The electrocyclic ring closure is is designated by blue arrows, and the ring opening by red arrows. Once again, the number of curved arrows that describe the bond reorganization is half the total number of electrons involved in the process.
In the first case, trans,cis,trans-2,4,6-octatriene undergoes thermal ring closure to cis-5,6-dimethyl-1,3-cyclohexadiene. The sterospecificity of this reaction is demonstrated by closure of the isomeric trans,cis,cis-triene to trans-5,6-dimethyl-1,3-cyclohexadiene, as noted in the second example.
This mode of reaction is favored by relief of ring strain, and the reverse ring closure (light blue arrows) is not normally observed. Photochemical ring closure can be effected, but the stereospecificity is opposite to that of thermal ring opening.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|