


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 31-5-2016
Date: 17-7-2019
Date: 18-7-2019
|
Acyl chlorides having at least one alpha-hydrogen undergo elimination of HCl on treatment with 3º-amine bases (1st equation below). The resulting carbon-carbon double bond has a cumulative relationship to the carbonyl double bond, and compounds of this kind are called ketenes. Elimination of vicinal dichlorides by reaction with zinc dust is also possible (2nd equation), and thermal dehydration of acetic acid generates the parent structure, named ketene (3rd equation).
|
1. R2CHCOCl + R'3N |
|
2. Cl3CCOCl + Zn (dust) |
|
3. CH3CO2H + AlPO4 & heat |
Ketenes are reactive intermediates which combine rapidly with nucleophiles to give carboxylic acid derivatives or, if no other reaction is possible, eventually dimerize (or polymerize). Some characteristic reactions of ketene are shown in the following diagram. The β-lactone structure at the lower left is a stable compound known as diketene.
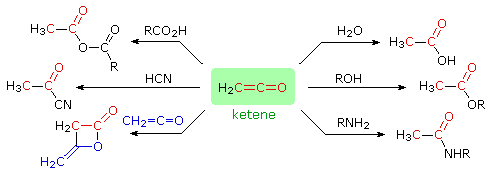



|
|
|
|
الصين.. طريقة لمنع تطور قصر النظر لدى تلاميذ المدارس
|
|
|
|
|
|
|
ماذا سيحدث خلال كسوف الشمس يوم السبت؟
|
|
|
|
|
|
|
قسم الشؤون الدينية يختتم محاضراته الرمضانية في صحن مرقد أبي الفضل العباس (عليه السلام)
|
|
|