


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 6-9-2019
Date: 14-11-2019
Date: 28-6-2019
|
The smallest such hydrocarbon is naphthalene. Naphthalene is stabilized by resonance. Three canonical resonance contributors may be drawn, and are displayed in the following diagram.
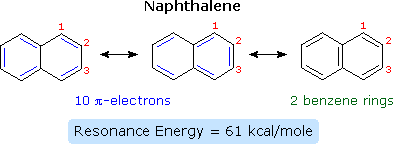
The two structures on the left have one discrete benzene ring each, but may also be viewed as 10-pi-electron annulenes having a bridging single bond. The structure on the right has two benzene rings which share a common double bond. From heats of hydrogenation or combustion, the resonance energy of naphthalene is calculated to be 61 kcal/mole, 11 kcal/mole less than that of two benzene rings (2 * 36). As expected from an average of the three resonance contributors, the carbon-carbon bonds in naphthalene show variation in length, suggesting some localization of the double bonds. The C1–C2 bond is 1.36 Å long, whereas the C2–C3 bond length is 1.42 Å. This contrasts with the structure of benzene, in which all the C–C bonds have a common length, 1.39 Å.
Naphthalene is more reactive than benzene, both in substitution and addition reactions, and these reactions tend to proceed in a manner that maintains one intact benzene ring. The following diagram shows three oxidation and reduction reactions that illustrate this feature. In the last example, catalytic hydrogenation of one ring takes place under milder conditions than those required for complete saturation (the decalin product exists as cis/trans isomers). Electrophilic substitution reactions take place more rapidly at C1, although the C2 product is more stable and predominates at equilibrium. Examples of these reactions is shown in below diagram. The kinetically favored C1 orientation reflects a preference for generating a cationic intermediate that maintains one intact benzene ring.
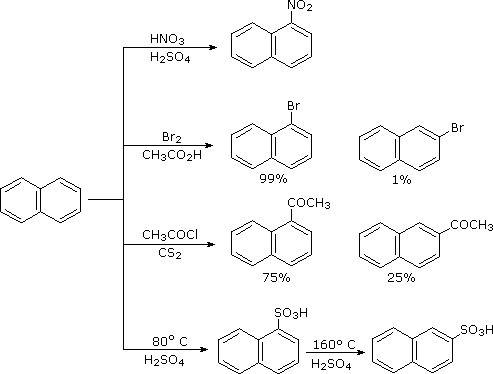
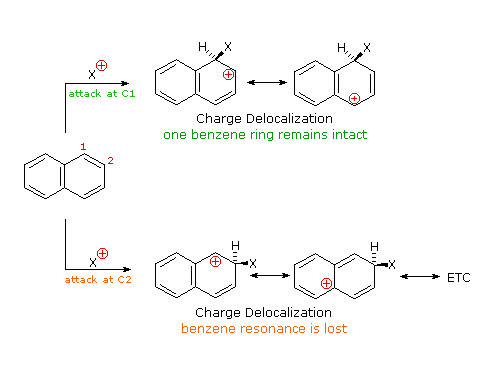



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|