


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-8-2017
Date: 11-9-2017
Date: 6-8-2017
|
n-BUTANE CHEMICALS
Like propane, n-butane is mainly obtained from natural gas liquids.
It is also a by-product from different refinery operations. Currently, the major use of n-butane is to control the vapor pressure of product gasoline. Due to new regulations restricting the vapor pressure of gasolines, this use is expected to be substantially reduced. Surplus n-butane could be isomerized to isobutane, which is currently in high demand for producing isobutene. Isobutene is a precursor for methyl and ethyl tertiary butyl ethers, which are important octane number boosters. Another alternative outlet for surplus n-butane is its oxidation to maleic anhydride. Almost all new maleic anhydride processes are based on butane oxidation. n-Butane has been the main feedstock for the production of butadiene. However, this process has been replaced by steam cracking hydrocarbons, which produce considerable amounts of by-product butadiene.
The chemistry of n-butane is more varied than that of propane, partly because n-butane has four secondary hydrogen atoms available for substitution and three carbon-carbon bonds that can be cracked at high temperatures:
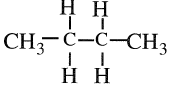
Like propane, the noncatalytic oxidation of butane yields a variety of products including organic acids, alcohols, aldehydes, ketones, and olefins. Although the noncatalytic oxidation of butane produces mainly aldehydes and alcohols, the catalyzed oxidation yields predominantly acids.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
قسم الشؤون الفكرية والثقافية يجري اختبارات مسابقة حفظ دعاء أهل الثغور
|
|
|