


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-4-2017
Date: 24-6-2017
Date: 18-7-2017
|
Strengths of Acids and Bases
The equilibria of Eqs. (1-1) and (1-2) correspond to the equilibrium constant expressions:
 (1.1)
(1.1)
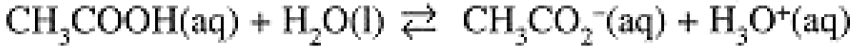 (1.2)
(1.2)
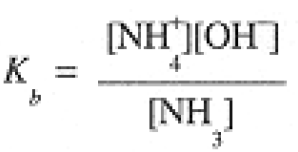 (1.3)
(1.3)
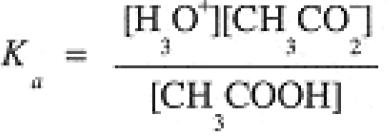 (1.4)
(1.4)
These expressions are often written in logarithmic form:
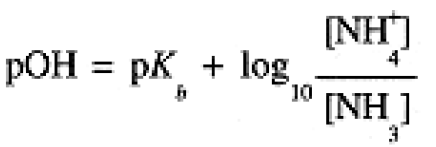 (1.5)
(1.5)
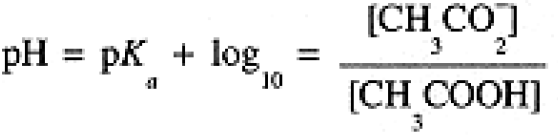 (1.6)
(1.6)
We can also think of NH4+ as an acid, so that in solutions of NH4Cl, we have the equilibrium
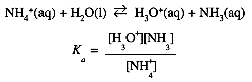
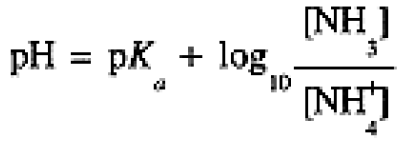 (1.7)
(1.7)
Adding eqs (1-5) and (1-7), we have
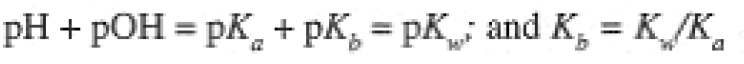 (1.8)
(1.8)
Equation (1-8) is a general result relating pKa of an acid with pKb of the conjugate base. The value of Ka or Kb (pKa or pKb) determines the extent of ionization of an acid or base. We think of strong acids or bases as completely ionized (or nearly so). This generally implies Ka or Kb greater than 1 (negative pKa or pKb). The pKa values for several acids are given in Table 1.1.
Many common acids are oxoacids, i.e., molecules with the central atom surrounded by =O (oxo) and –OH (hydroxo) groups. Perchloric (HClO4), chloric (HClO3), chlorous (HClO2), and hypochlorous (HClO) acids can be written ClO3OH), ClO2(OH), ClO(OH), and ClOH, respectively, to emphasize the distinction between oxo and hydroxo oxygens. The acid strength increases with the number of oxo O's. The increased acidity results from the delocalization of negative charge in the anions as shown in Figure 1.1 for the Cl oxoanions. The average formal charge on oxygen is -1/4 in ClO4-, -1/3 in ClO3-, -1/2 in ClO2-, and -1 in ClO-.
Table 1.1. pKa's of Some Common Acids
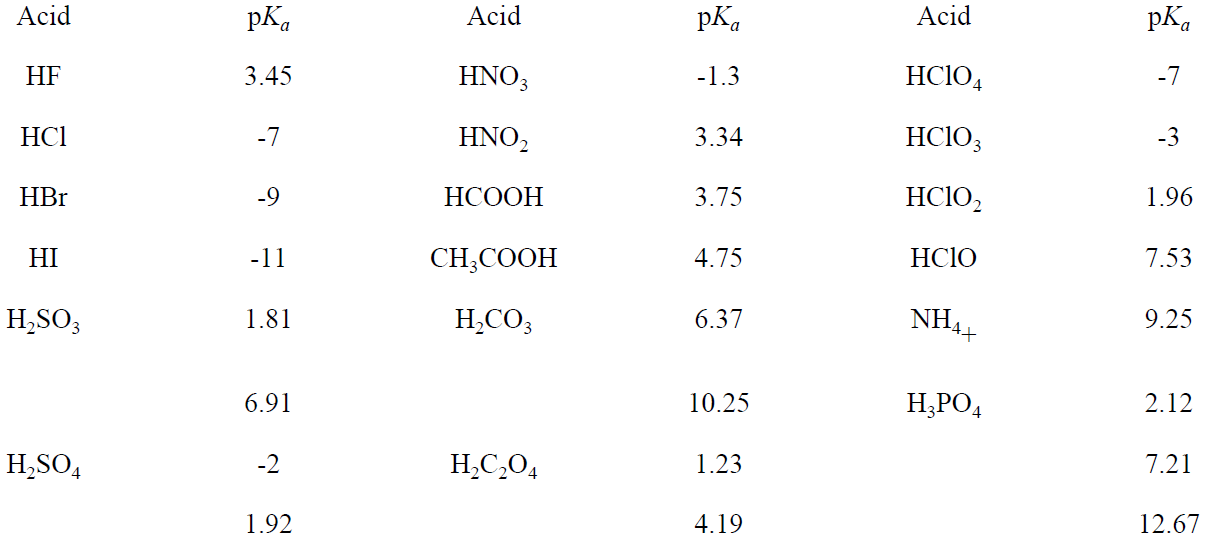

Figure 1.1. Resonance structures of the perchlorate, chlorate, chlorite, hypochlorite, formate, and nitrite ions.
Five of the acids listed in Table 1.1 are polyprotic acids, i.e., acids with more than one acidic H. Forexample, phosphoric acid ionizes in three steps:
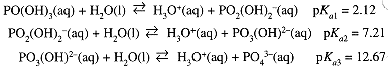
The acidity of the neutral acid is in the range expected for an oxoacid with one oxo O. In the second and third steps, phosphoric acid is a much weaker acid. In general, we find that pKa increases by about 5 for each successive ionization step for a polyprotic acid. An exception to this rule is oxalic acid, H2C2O4 = HOOCCOOH, where the acidic -OH groups are bound to different C atoms and behave more nearly independently.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|