


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-1-2020
Date: 20-1-2020
Date: 20-1-2020
|
Radical Additions to Alkenes: Chain-Growth Polymers
.A polymer is simply a large—sometimes very large—molecule, built up by repetitive bonding of many smaller molecules, called monomers. Nature makes wide use of biological polymers. Cellulose, for instance, is a polymer built of repeating glucose monomer units; proteins are polymers built of repeating amino acid monomers; and nucleic acids are polymers built of repeating nucleotide monomers.
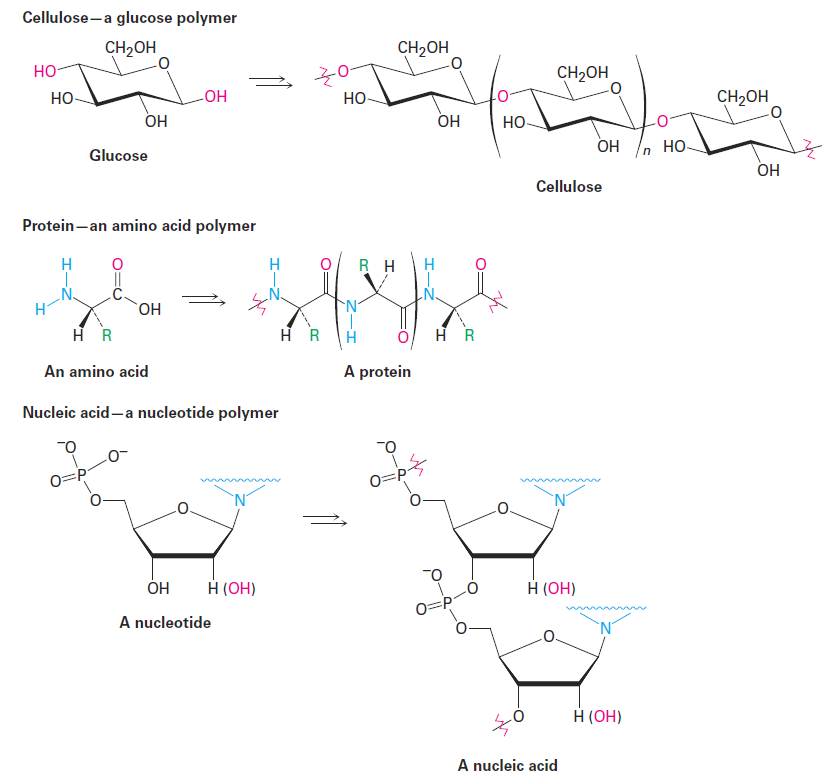
Synthetic polymers, such as polyethylene, are much simpler chemically than biopolymers, but there is still a great diversity to their structures and properties, depending on the identity of the monomers and on the reaction conditions used for polymerization. The simplest synthetic polymers are those that result when an alkene is treated with a small amount of a suitable catalyst.
Ethylene, for example, yields polyethylene, an enormous alkane that may have a molecular weight up to 6 million amu and may contain as many as 200,000 monomer units incorporated into a gigantic hydrocarbon chain. Worldwide production of polyethylene is approximately 80 million metric tons per year.
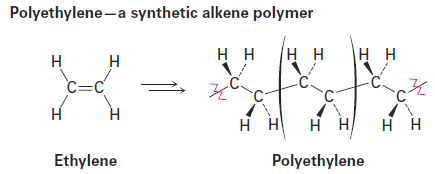
Polyethylene and other simple alkene polymers are called chaingrowth polymers because they are formed in a chain-reaction process in which an initiator adds to a carbon–carbon double bond to yield a reactive intermediate. The intermediate then reacts with a second molecule of monomer to yield a new intermediate, which reacts with a third monomer unit, and so on.
Historically, ethylene polymerization was carried out at high pressure (1000–3000 atm) and high temperature (100–250 °C) in the presence of a radical initiator such as benzoyl peroxide, although other catalysts and reaction conditions are now used. The key step is the addition of a radical to the ethylene double bond, a reaction similar in many respects to what takes place in the addition of an electrophile. When sketching the mechanism, recall that a curved half-arrow, or “fishhook” , is used to show the movement of a single electron, as opposed to the full curved arrow used to show the movement of an electron pair in polar reactions.
• Initiation The polymerization reaction is initiated when a few radicals are generated on heating a small amount of benzoyl peroxide catalyst to break the weak O - O bond. The initially formed benzoyloxy radical loses CO2 and gives a phenyl radical (Ph·), which adds to the C=C bond of ethylene to start the polymerization process. One electron from the ethylene double bond pairs up with the odd electron on the phenyl radical to form a new C - C bond, and the other electron remains on carbon.
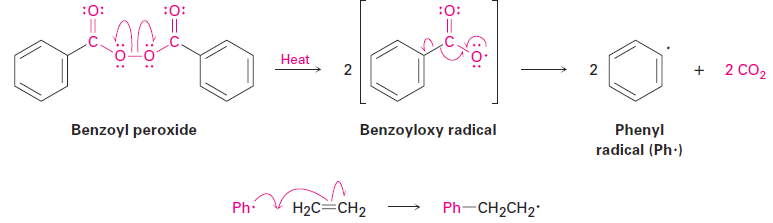
• Propagation Polymerization occurs when the carbon radical formed in the initiation step adds to another ethylene molecule to yield another radical. Repetition of the process for hundreds or thousands of times builds the polymer chain.

• Termination The chain process is eventually ended by a reaction that consumes the radical. The combination of two growing chains is one possible chain-terminating reaction.

Ethylene is not unique in its ability to form a polymer. Many substituted ethylenes, called vinyl monomers, also undergo polymerization to yield polymers with substituent groups regularly spaced on alternating carbon atoms along the chain. Propylene, for example, yields polypropylene, and styrene yields polystyrene.
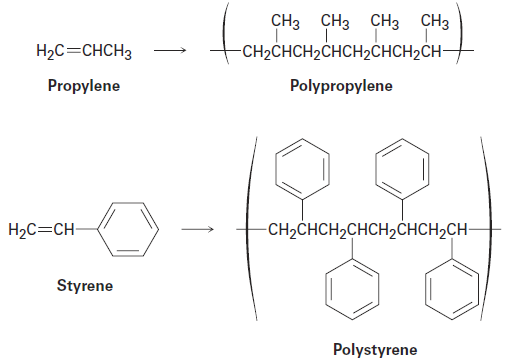
When an unsymmetrically substituted vinyl monomer such as propylene or styrene is polymerized, the radical addition steps can take place at either end of the double bond to yield either a primary radical intermediate (RCH2·) or a secondary radical (R2CH·). Just as in electrophilic addition reactions, however, we find that only the more highly substituted, secondary radical is formed.
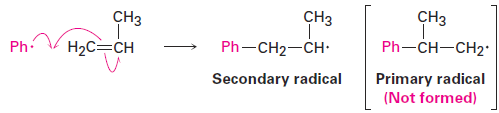
Table 1.1 shows some commercially important alkene polymers, their uses, and the vinyl monomers from which they are made. Table 1.1 Some Alkene Polymers and Their Uses




|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|