


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 12-7-2019
Date: 11-7-2016
Date: 28-8-2018
|
The Hammond Postulate
Let’s summarize what we’ve learned of electrophilic addition reactions to this point:
• Electrophilic addition to an unsymmetrically substituted alkene gives the more highly substituted carbocation intermediate. A more highly substituted carbocation forms faster than a less highly substituted one and, once formed, rapidly goes on to give the final product.
• A more highly substituted carbocation is more stable than a less highly substituted one. That is, the stability order of carbocations is tertiary.
secondary > primary > methyl.
What we have not yet seen is how these two points are related. Why does the stability of the carbocation intermediate affect the rate at which it’s formed and thereby determine the structure of the final product? After all, carbocation stability is determined by the free-energy change ΔG°, but reaction rate is determined by the activation energy ΔG‡. The two quantities aren’t directly related. Although there is no simple quantitative relationship between the stability of a carbocation intermediate and the rate of its formation, there is an intuitive relationship. It’s generally true when comparing two similar reactions that the more stable intermediate forms faster than the less stable one. The situation is shown graphically in Figure 1.1, where the energy profile in part (a) represents the typical situation, as opposed to the profile in part (b). That is, the curves for two similar reactions don’t cross one another.
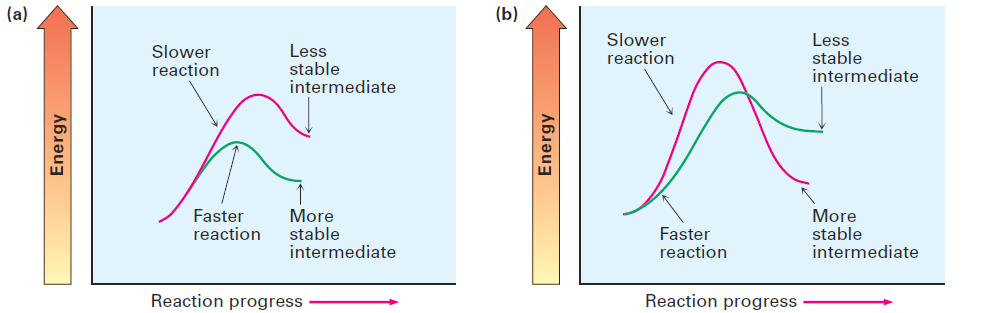
Figure 1.1 Energy diagrams for two similar competing reactions. In (a), the faster reaction yields the more stable intermediate. In (b), the slower reaction yields the more stable intermediate. The curves shown in (a) represent the typical situation. Called the Hammond postulate, the explanation of the relationship between reaction rate and intermediate stability goes like this: Transition states represent energy maxima. They are high-energy activated complexes that occur transiently during the course of a reaction and immediately go on to a more stable species.
Although we can’t actually observe transition states because they have no finite lifetime, the Hammond postulate says that we can get an idea of a particular transition state’s structure by looking at the structure of the nearest stable species. Imagine the two cases shown in Figure 1.2, for example. The reaction profile in part (a) shows the energy curve for an endergonic reaction step, and the profile in part (b) shows the curve for an exergonic step.
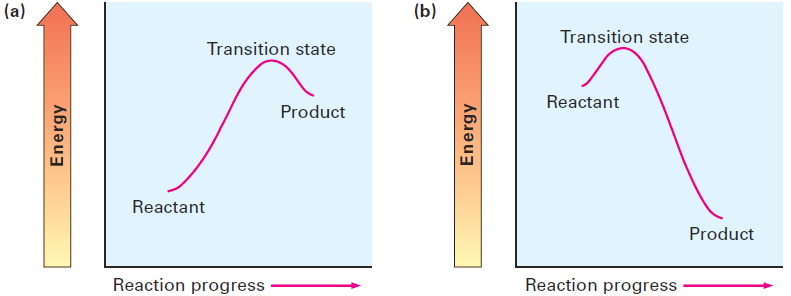
Figure 1.2 Energy diagrams for endergonic and exergonic steps. (a) In an endergonic step, the energy levels of transition state and product are closer. (b) In an exergonic step, the energy levels of transition state and reactant are closer.
In an endergonic reaction (Figure 1.2a), the energy level of the transition state is closer to that of the product than that of the reactant. Since the transition state is closer energetically to the product, we make the natural assumption that it’s also closer structurally. In other words, the transition state for an endergonic reaction step structurally resembles the product of that step. Conversely, the transition state for an exergonic reaction (Figure 1.2b) is closer energetically, and thus structurally, to the reactant than to the product. We therefore say that the transition state for an exergonic reaction step structurally resembles the reactant for that step.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تقدم دعوة إلى كلية مزايا الجامعة للمشاركة في حفل التخرج المركزي الخامس
|
|
|