


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 2-3-2018
Date: 25-2-2018
Date: 25-2-2018
|
Filtering and Washing Precipitates
The steps in filtering an analytical precipitate are decantation, washing, and transfer. In decantation, as much supernatant liquid as possible is passed through the filter while the precipitated solid is kept essentially undisturbed in the beaker where it was formed. This procedure speeds the overall filtration rate by delaying the time at which the pores of the filtering medium become clogged with precipitate. A stirring rod is used to direct the flow of the decanted liquid (Figure 1.1a).
When flow ceases, the drop of liquid at the end of the pouring spout is collected with the stirring rod and returned to the beaker. Wash liquid is next added to the beaker and thoroughly mixed with precipitate. The solid is allowed to settle, and then this liquid is also decanted through the filter. Several such washings may be required, depending on the precipitate.
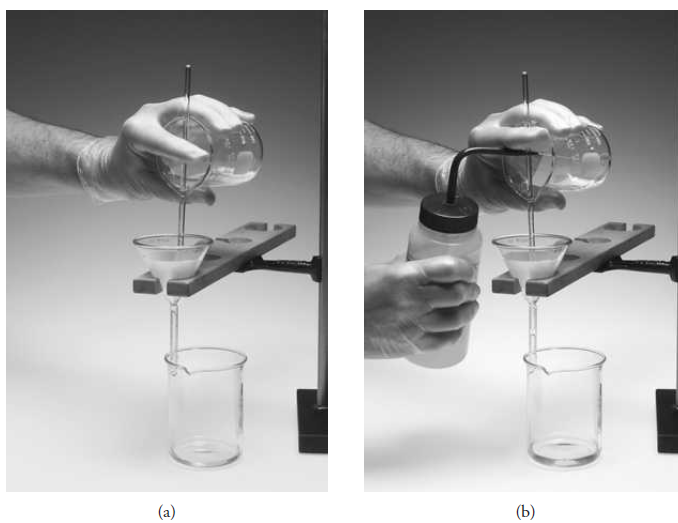
Figure 1.1 Washing by decan- (a) tation. (b) Transferring the precipitate.
Most washing should be carried out before the bulk of the solid is transferred. This technique results in a more thoroughly washed precipitate and a more rapid filtration.
The transfer process is illustrated in Figure 1.1b. The bulk of the precipitate is moved from beaker to filter by directed streams of wash liquid. As in decantation and washing, a stirring rod provides direction for the flow of material to the filtering medium. The last traces of precipitate that cling to the inside of the beaker are dislodged with a rubber policeman, which is a small section of rubber tubing that has been crimped on one end. The open end of the tubing is fitted onto the end of a stirring rod and is wetted with wash liquid before use. Any solid collected with it is combined with the main portion on the filter. Small pieces of ashless paper can be used to wipe the last traces of hydrous oxide precipitates from the wall of the beaker. These papers are ignited along with the paper that holds the bulk of the precipitate.
Many precipitates possess the exasperating property of creeping, or spreading over a wetted surface against the force of gravity. Filters are never filled to more than three quarters of capacity to prevent the possible loss of precipitate through creeping. The addition of a small amount of nonionic detergent, such as Triton X-100, to the supernatant liquid or wash liquid can help minimize creeping. A gelatinous precipitate must be completely washed before it is allowed to dry. These precipitates shrink and develop cracks as they dry. Further additions of wash liquid simply pass through these cracks and accomplish little or no washing.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|