


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 11-4-2017
Date: 27-3-2017
Date: 26-11-2020
|
Nuclear Forces
In the Bohr model of the atom, the nucleus consists of positively-charged protons and electricallyneutral neutrons. Since both protons and neutrons exist in the nucleus, they are both referred to as nucleons. One problem that the Bohr model of the atom presented was accounting for an attractive force to overcome the repulsive force between protons.
Two forces present in the nucleus are (1) electrostatic forces between charged particles and (2) gravitational forces between any two objects that have mass. It is possible to calculate the magnitude of the gravitational force and electrostatic force based upon principles from classical physics.
Newton stated that the gravitational force between two bodies is directly proportional to the masses of the two bodies and inversely proportional to the square of the distance between the bodies. This relationship is shown in the equation below.
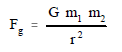
where:
Fg = gravitational force (newtons)
m1 = mass of first body (kilograms)
m2 = mass of second body (kilograms)
G = gravitational constant (6.67 x 10 -11 N-m2/kg2)
r = distance between particles (meters)
The equation illustrates that the larger the masses of the objects or the smaller the distance between the objects, the greater the gravitational force. So even though the masses of nucleons are very small, the fact that the distance between nucleons is extremely short may make the gravitational force significant. It is necessary to calculate the value for the gravitational force and compare it to the value for other forces to determine the significance of the gravitational force in the nucleus. The gravitational force between two protons that are separated by a distance of 10 -20 meters is about 10 -24 newtons.
Coulomb's Law can be used to calculate the force between two protons. The electrostatic force is directly proportional to the electrical charges of the two particles and inversely proportional to the square of the distance between the particles. Coulomb's Law is stated as the following equation.

where:
Fe = electrostatic force (newtons)
K = electrostatic constant (9.0 x 109 N-m2/C2)
Q1 = charge of first particle (coulombs)
Q2 = charge of second particle (coulombs)
r = distance between particles (meters)
Using this equation, the electrostatic force between two protons that are separated by a distance of 10 -20 meters is about 10 12 newtons. Comparing this result with the calculation of the gravitational force (10-24 newtons) shows that the gravitational force is so small that it can be neglected.
If only the electrostatic and gravitational forces existed in the nucleus, then it would be impossible to have stable nuclei composed of protons and neutrons. The gravitational forces are much too small to hold the nucleons together compared to the electrostatic forces repelling the protons. Since stable atoms of neutrons and protons do exist, there must be another attractive force acting within the nucleus. This force is called the nuclear force.
The nuclear force is a strong attractive force that is independent of charge. It acts equally only between pairs of neutrons, pairs of protons, or a neutron and a proton. The nuclear force has a very short range; it acts only over distances approximately equal to the diameter of the nucleus (10 -13 cm). The attractive nuclear force between all nucleons drops off with distance much faster than the repulsive electrostatic force between protons.
TABLE 1: Forces Acting in the Nucleus
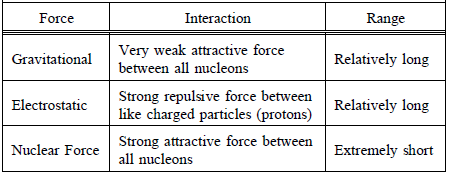
In stable atoms, the attractive and repulsive forces in the nucleus balance. If the forces do not balance, the atom cannot be stable, and the nucleus will emit radiation in an attempt to achieve a more stable configuration.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
بالصور: تزامنا مع ختام فعالياته.. ممثل المرجعية العليا يشارك في المحفل القرآني المركزي في الصحن الحسيني الشريف
|
|
|