


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 26-4-2017
Date: 25-2-2018
Date: 7-5-2017
|
Reaction stoichiometry
When you understand the weight relationships in a chemical reaction, you can do some stoichiometry problems. Stoichiometry refers to the mass relationship in chemical equations.
When you get ready to work stoichiometry types of problems, you must start with a balanced chemical equation. If you don’t have it to start with, go ahead and balance the equation.
Look at my favorite reaction — you guessed it — the Haber process:
N2(g) + 3 H2(g) ↔ 2 NH3(g)
Suppose that you want to know how many grams of ammonia can be produced from the reaction of 75.00 grams of nitrogen with excess hydrogen. The mole concept is the key. The coefficients in the balanced equation are not only the number of individual atoms or molecules but also the number of moles:
N2(g) + 3 H2(g) ↔ 2 NH3(g)
1 mole + 3 moles ↔ 2 moles
1 mol(28.014 g/mol) + 3 mol(2.016 g/mol) = 2 mol(17.031g/mol)
First, convert the 75.00 grams of nitrogen to moles of nitrogen. Then use the ratio of the moles of ammonia to the moles of nitrogen from the balanced equation to convert to moles of ammonia. Finally, take the moles of ammonia and number to grams. The equation looks like this:
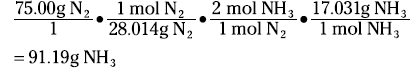
A stoichiometric ratio — such as mol NH3/mol N2 — enables you to convert from the moles of one substance in a balanced chemical equation to the moles of another substance.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|