


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 6-7-2017
Date: 6-7-2017
Date: 5-3-2019
|
Comparing covalent bonds with other bonds
The properties of ionic and covalent compounds are different. Table 1-1 shows how the compounds compare.
Table 1-1 Properties of Ionic and Covalent Compounds
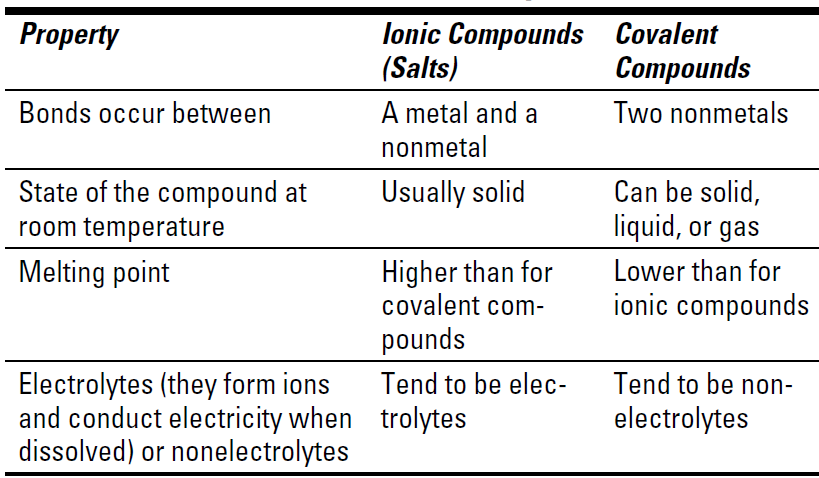
I know just what you’re thinking: If metals react with nonmetals to form ionic bonds, and nonmetals react with other nonmetals to form covalent bonds, do metals react with other metals? The answer is yes and no. Metals don’t really react with other metals to form compounds. Instead, the metals combine to form alloys, solutions of one metal in another.
But there is such a situation as metallic bonding, and it’s present in both alloys and pure metals. In metallic bonding, the valence electrons of each metal atom are donated to an electron pool, commonly called a sea of electrons, and are shared by all the atoms in the metal. These valence electrons are free to move throughout the sample instead of being tightly bound to an individual metal nucleus. The ability of the valence electrons to flow throughout the entire metal sample is why metals tend to be conductors of electricity and heat.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
بحضور الأمين العام للعتبة الحسينية.. قسم دار القرآن الكريم في العتبة الحسينية يكرم الكوادر المتطوعة في الختمة القرآنية الرمضانية
|
|
|