


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 17-4-2021
Date: 9-3-2021
Date: 28-11-2015
|
Hemidesmosomes
Hemidesmosomes are cell junctions that are recognized by their ultrastructural appearance (Fig. 1). Located at the basal cell surface, their most prominent feature in cross section is a dense outer plaque closely applied to the inner surface of the plasma membrane (1). The outer plaque is separated by a less dense region from an inner dense plaque, from which intermediate filaments extend into the cytoplasm. The whole structure forms a circular membrane domain of no more than 0.5 micrometres in diameter and approximately 150 nm deep from the plasma membrane to the inner surface of the inner plaque. The extracellular face of the hemidesmosome is in contact with the lamina lucida of the basement membrane. This region is characterized by the presence of fine anchoring filaments that extend from the plasma membrane across the lamina lucida to the lamina densa. In some sections a linear density called the sub-basal dense plate is seen in the lamina lucida, closely associated with the extracellular face of the hemidesmosomal plasma membrane. Particularly in the epidermis and amnion, the matrix beneath the basement membrane contains numerous banded structures, called anchoring fibrils, which insert into the lamina densa. In favorable sections, filamentous continuity between the anchoring fibrils and the anchoring filaments can be observed. This means that there is continuity of structure in epidermis from the dermis right through to the intermediate filament cytoskeleton of the epidermal cells, in which the hemidesmosome itself provides the transmembrane connection. Such continuity appears to be essential for strong adhesive binding of the epidermis to the dermis, because genetic defects leading to absence or abnormality of anchoring fibrils, anchoring filaments, hemidesmosomes, or basal cell keratin filaments give rise to blistering diseases involving loss of adhesion between the epidermis and the dermis. Although hemidesmosomes appear to resemble half desmosomes, they are in fact quite different structures, with a unique and distinct molecular composition (2).
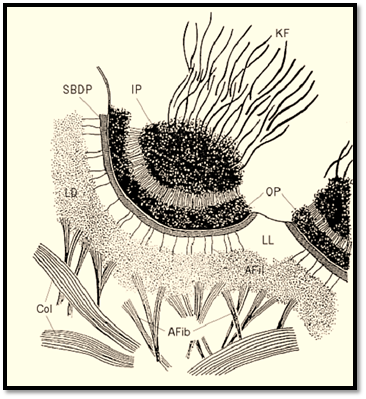
Figure 1. Drawing of an epidermal hemidesmosome seen by electron microscopy. Afib = anchoring fibril; Afil = anchoring filament; Col-dermal collagen fiber; IP = inner plaque; KF = keratin filaments; LD = lamina densa; LL = lamina lucida; SBDP = sub–basal dense plate.
The major molecular components of hemidesmosomes are shown in Figure 2 . The principal adhesion molecule is a6b4 integrin (3), a heterodimer that is extremely abundant on the basal surface of epidermal cells. The b4 subunit is unique among integrin b subunits in having an extremely long cytoplasmic domain of approximately 1000 amino acid residues. The extracellular domains of the integrin subunits lie in the lamina lucida of the basement membrane, and their cytoplasmic domains in the hemidesmosomal plaque. The other adhesion molecule is bullous pemphigoid antigen 2 ) BPAG2 or BP180), a Type 2 membrane protein whose amino-terminus lies within the desmosomal plaque and the carboxy-terminus in the basement membrane (4). BPAG2 is also referred to as collagen XVII, because it has collagenous repeats in its extracellular domain. a6b4 integrin binds to the truncated laminin molecule, laminin 5, which is covalently complexed with laminin 6 or laminin 7 (5). Laminin 5, together with the extracellular domain of BP180, constitutes the anchoring filaments. Laminin 6 and 7 interact with other laminins in the basement membrane and also with nidogen, which links them to other basement membrane components. In addition, they bind to the specific component of anchoring fibrils, collagen VII (6). The anchoring fibrils descend from the lamina densa of the basement membrane into the underlying dermis. The principal plaque components of the hemidesmosome are bullous pemphigoid antigen 1 (BPAG1 or BP230) and plectin (7, 8). Both are members of the plakin family, which also includes desmoplakin, envoplakin, and periplakin of the desmosome . BP230 and plectin are believed to be involved in linking between the hemidesmosomal plaque and the intermediate filaments. Although not shown in the diagram, direct interaction between plectin and a6b4 integrin has been demonstrated (9).
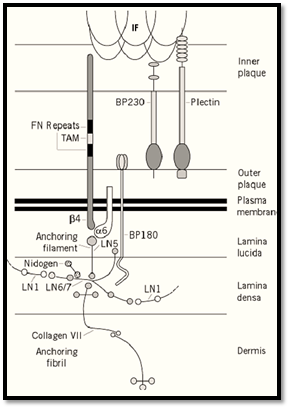
Figure 2. Diagrammatic impression of the molecular composition of a hemidesmosome.
A number of human diseases have been extremely important in elucidating the composition and function of hemidesmosmes. In the autoimmune blistering disease bullous pemphigoid, antibodies against BPAG1 and 2 are commonly found. Those against an extracellular juxta membrane epitope of BPAG2 have been shown to be pathogenic (10). A number of genetic diseases, collectively known as epidermolysis bullosa (EB), affect basal cells of epidermis and epidermal-basement membrane adhesion. EB simplex involves the basal cell keratins, K5 and K14 (see Keratins). Such mutations disrupt the assembly of the basal cell intermediate filaments and result in cell breakage at a level above the hemidesmosomes (11). The junctional form of EB (EBJ) involves mutations in the a, b, or g chains of laminin 5 or in a6b 4 integrin (12). This is a lethal disease resulting in epidermal detachment at the level of the basement membrane. EB dystrophica results from mutations of the anchoring fibril component Type VII collagen (13). Anchoring fibrils fail to form, and the epidermis becomes detached below the level of the basement membrane. The importance of plectin in hemidesmosomes has been revealed by the disease EB with muscular dystrophy (14). Here attachment of intermediate filaments from the hemidesmosomal plaque occurs in basal epidermal cells, resulting in epidermal blistering. This is followed later in life by muscular weakening, arising from absence of plectin from the submembrane cytoskeleton of skeletal muscle fibers. Null mutations of a6b4 integrin, plectin, and BP230 produce phenotypes consistent with the defects found in human disease (15-18). This work suggests that BPAG1 forms a similar function to plectin in attaching intermediate filaments to the plaque. The BPAG1 null nutation revealed an additional function for this molecule in the nervous system (17). BPAG1–/– mice develop severe dystonia, resulting in abnormal limb positioning and loss of involvement through muscle and nerve regeneration. This is reminiscent of a hereditary neurodegenerative disease of mice called dystonia musculorum (dt/dt). dt is allelic with BP230. It appears that alternative splicing of the amino-terminus of BPAG1 in the nervous system promotes interaction with actin, and its absence results in disorganisation of the neuronal cytoskeleton (19). It is noteworthy that the dermal epidermal junction requires extremely strong adhesion of cells to the extracellular matrix. This may be why hemidesmosomes have two adhesion molecules and two molecules to bridge between the plaque and the intermediate filaments.
References
1. J. E. Ellison and D. R. Garrod (1984) J. Cell Sci. 72, 163–172.
2. P. K. Legan et al. (1992) Bioessays 14, 385–393.
3. A. Sonnenberg et al. (1991) J. Cell Biol. 113, 907–917.
4. Y. Hirako et al. (1996) J. Biol. Chem. 271, 13739–13745.
5. M. F. Champliaud (1996) J. Cell Biol. 132, 1189–1198.
6. H. P. Bächinger et al. (1990) J. Biol. Chem. 265, 10095–10101.
7. D. H. Klatte et al. (1989) J. Cell Biol. 109, 3377–3390.
8. G. Wiche et al. (1983) J. Cell Biol. 97, 887–901.
9. C. M. Neissen (1997) J. Cell Sci. 110, 1705–1716.
10. Z. Liu et al. (1993) J. Clin. Invest. 92, 2480–2488.
11. W. H. I. McLean and E. B. Lane (1995) Curr. Opin. Cell Biol. 7, 118–125.
12. R. A. J. Eady and M. G. Dunhill (1994) Arch. Dermatol. Res. 287, 2–9.
13. A. M. Christiano et al. (1994) Proc. Natl. Acad. Sci. USA 91, 3549–3553.
14. W. H. I. McLean et al. (1996) Genes. Dev. 10, 1724–1735.
15. E. Georges-Labouesse et al. (1996) Nature Genet. 13, 370–373.
16. R. Van der Neut et al. (1996) Nature Genet. 13, 366–369.
17. K. Andrä et al. (1997) Genes Dev. 11, 3143–3156.
18. L. Guo et al. (1995) Cell 81, 233–243.
19. Y. Yang et al. (1996) Cell 86, 655–665.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|