


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 27-4-2016
Date: 30-3-2021
Date: 23-5-2016
|
Guanine Nucleotide Exchange Factors
Guanine nucleotide exchange factors (GEFs) are a structurally diverse group of proteins that are defined by a common function: catalyzing the exchange of bound GDP for GTP on GTP-binding regulatory proteins. They act on target proteins as diverse as initiation factors and elongation factors of translation, the ras-like monomeric GTP-binding proteins, and the heterotrimeric G proteins. Because the GTP-binding target proteins are activated when GTP binds, the GEFs are stimulatory regulators. The GEFs are also referred to as GDP dissociation stimulators (GDSs), GDP release factors (GRFs), and GDP/GTP exchange catalysts.
The prototypical GEF is the bacterial translational elongation factor EF-Ts, which is the GEF for EF-Tu. GEFs related to EF-Ts are found in all organisms. GEFs for the heterotrimeric G proteins are cell-surface receptors that initiate G-protein signaling. They are based on a hydrophobic core of seven membrane-spanning a-helices that binds ligand on the extracellular face and binds G proteins on the cytoplasmic face. GEFs for the small, monomeric GTP-binding regulatory proteins in eukaryotes also fall into classes specific for their targets. While there is no globally identifiable GEF consensus sequence or protein structure, GEFs for a particular class of GTP-binding proteins usually share clear sequence homology, although GEFs are frequently multi-domain proteins that combine a GEF domain with domains that fulfill other signaling or protein–protein interaction functions. GEFs may be constitutively active, as are the GEFs for the translation factors. Alternatively, they may be regulated by ligand binding, by phosphorylation, by binding of yet other regulatory proteins, or by recruitment to their sites of action when they or their anchors are phosphorylated.
Despite the diversity of their structures and targets, GEFs catalyze GDP/GTP exchange by a common mechanism, shown below (where G is the target GTP-binding protein: (
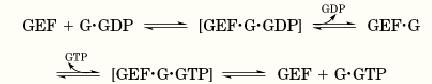
Formally, binding of a GEF to its target protein decreases the affinity of the target for guanine nucleotides: It apparently converts the nucleotide binding site to a more “open” configuration. When the GEF binds, dissociation of GDP is accelerated, leaving the GEF bound to the unliganded target. Binding of guanine nucleotide to the target protein must reciprocally decrease its affinity for the GEF, and cytoplasmic GTP is in large excess over GDP, so GTP binds and displaces GEF by the same mechanism. Release of the GEF after it has promoted GDP/GTP exchange allows a single GEF molecule to act sequentially on multiple molecules of target protein. Thus GEFs are true catalysts of exchange: They accelerate GDP/GTP exchange but do not alter the equilibrium balance of bound GTP and GDP.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|