
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-1-2016
Date: 4-10-2017
Date: 29-3-2016
|
PHASE TWO CHANGES
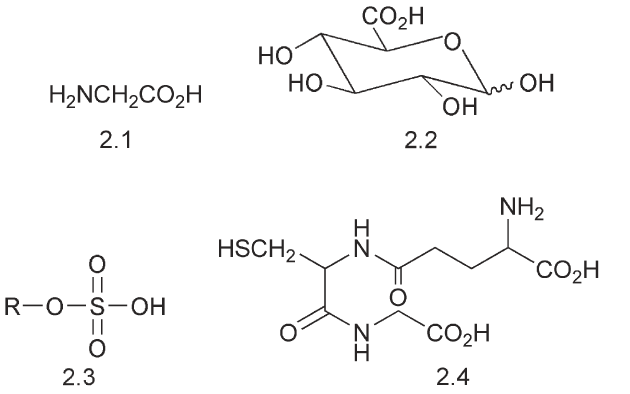
The phase two changes involve linking a water-solubilizing group to the drug. The common groups are glucuronic acid 2.2, which is provided by uridine-5-diphosphate a-D-glucuronic acid, the sulfate 2.3, which is provided by 30-phosphoadenosine-50-phosphonosulfate and the amino acids, glycine 2.1 and taurine. An important conjugation utilizes the tripeptide glutathione 2.4.
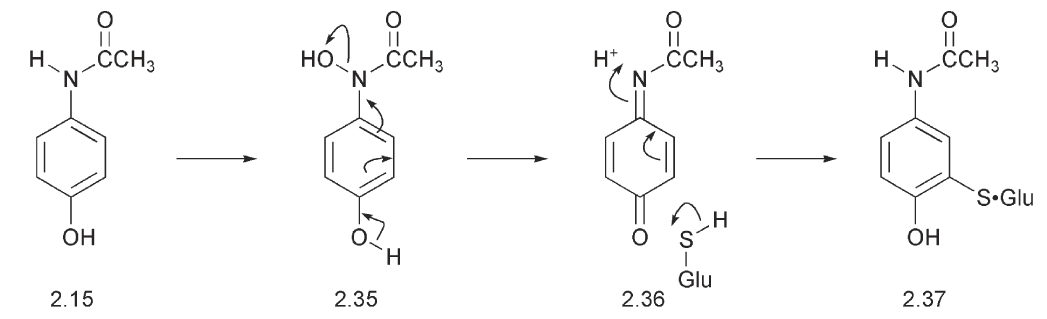
This possesses a thiol, which is a powerful nucleophile that can add to electron-deficient carbon atoms. Since electron-deficient centres can be created by oxidative processes and might otherwise be attacked by free amino groups of nucleic acids and proteins, the glutathione conjugation serves to protect the liver from damage. An example is provided by the metabolism of paracetamol. Oxidation of paracetamol takes place on the nitrogen with the formation of an N–OH 2.35 and this leads, after elimination to the quinone-imine 2.36. The latter is very electron-deficient and readily adds nucleophiles such as the NH2 from a protein. The consequence of this addition is that the paracetamol becomes bound to the liver causing liver damage. Glutathione 2.4 provides protection against this by adding to the quinone imine 2.36 to give a conjugate 2.37. If there is insufficient glutathione in the liver and the amount available is exhausted as in a paracetamol overdose, liver damage with potentially fatal consequences, ensues. Treatment involves the rapid administration of a thiol containing drug such as methionine or acetylcysteine.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
ملاكات العتبة العباسية المقدسة تُنهي أعمال غسل حرم مرقد أبي الفضل العباس (عليه السلام) وفرشه
|
|
|