


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 7-3-2016
Date: 14-3-2016
Date: 8-3-2016
|
Campylobacter
1. Introduction
Campylobacter is one of the major causes of diarrhea in humans and C. jejuni subsp. jejuni and C. coli are the species most frequently associated with acute foodborne gastroenteritis. C. lari and C. upsaliensis are also recognized as primary pathogens, but have not been isolated with the same frequency (WHO, 2000). In the United States and the United Kingdom, C. jejuni subsp. jejuni accounts for more than 90% of the strains isolated from clinical specimens (Tauxe et al., 1988, Stanley and Jones, 2003, Gupta et al., 2004).
Food-borne diseases caused by C. jejuni subsp. jejuni include gastroenteritis, septicemia, meningitis, abortion and the Guillain-Barré Syndrome (GBS). GBS is classified by the International Commission on Microbiological Specifications for Foods (ICMSF, 2002) into risk group IB: “diseases of severe hazard for restricted population; life threatening or resulting in substantial chronic sequelae or presenting effects of long duration”.
2. Taxonomy
The main characteristics of the Campylobacter species are summarized in Table 1.
Campylobacter belongs to the family Campylobacteriaceae, which includes actively growing cells generally curved or spiral-shaped (Vandamme et al., 2005a). Cells of most Campylobacter species are slender, spirally curved rods, Gram negative, non-spore-forming, which may have one or more spirals. They may also appear S-shaped and gull-winged when two cells form short chains. Cells in old cultures may form spherical or coccoid bodies. Most species are motile with a characteristic corkscrew-like motion by means of a single polar unsheated flagellum (Vandamme et al., 2005b).
Microaerophilic with a respiratory type of metabolism, they require low oxygen concentrations (3–15%) and 3 to 5% of CO2 for growth. Blood or serum stimulate growth, but are not indispensable. The energy metabolism of Campylobacter is oxidative and most strains are oxidase-positive, except for C. gracilis and some strains of C. concisus and C. showae. They do not use carbohydrates as source of energy, which is always derived from the oxidation of amino acids or intermediate compounds of the tricarboxylic acid cycle (Vandamme et al., 2005b).
They are inactive in most conventional biochemical tests, and do not produce acids or neutral metabolic end-products. They do not hydrolyze gelatin, casein, starch or tyrosin and are Methyl Red (MR) and Voges-Proskauer (VP) negative. Some species possess arylsulphatase activity, but do not produce lecithinase or lipase activity. Most Campylobacter species reduce nitrate (Vandamme et al., 2005b).
They grow in the 35–37ºC range, but not at 4ºC and most do also not grow at 25ºC. The optimal temperature varies in the 30–42ºC range. Stock cultures can be maintained under microaerobic conditions by weekly transfer onto common blood agar bases. Addition of blood to media may increase survival. For prolonged storage lyophilization or freezing at minus 80°C (with 10% glycerol or dimethyl sulfoxide as cryoprotectants) should be used (Vandamme et al., 2005b).
Thermotolerant campylobacters. The Campylobacter species associated with diseases transmitted by foods (C. jejuni, C. coli, C. lari and C. upsaliensis) form a distinct sub cluster within the Campylobacter genus, and are called thermotolerant (Vandamme et al., 2005b). These species typically have an optimal growth temperature in the 42–43°C range (Pearson and Healing, 1992) and do not grow below 30°C (Stanley and Jones, 2003).
Table.1 Biochemical and growth characteristics of the species and subspecies of Campylobacter (Vandamme et al., 2005)a
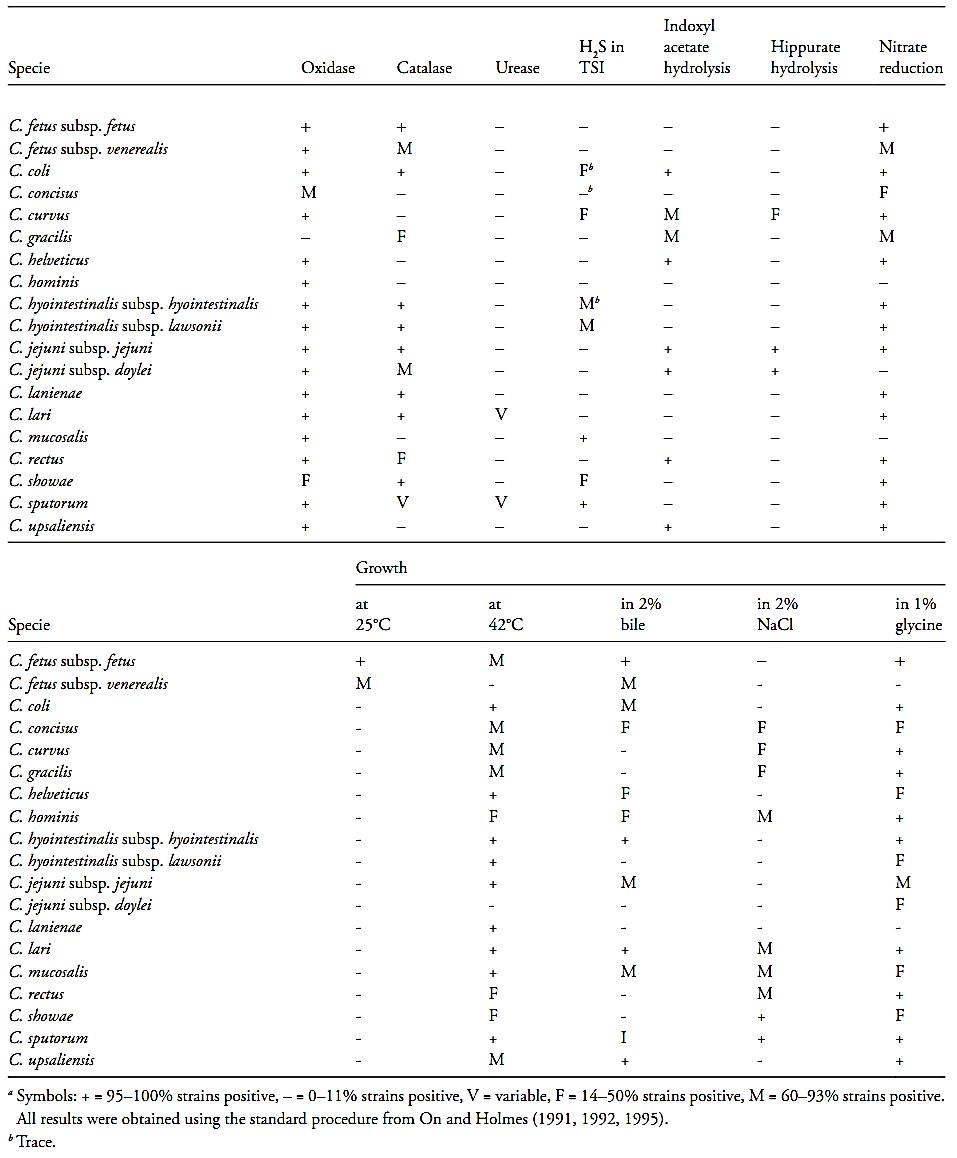
C. jejuni cells are small, tightly coiled spiral or S-shaped and transform rapidly to coccoid forms with age or exposure to toxic concentration of oxygen. Strains are motile by means of a single polar flagellum which seems to be an important virulence factor, necessary for colonization of the intestinal tract. Two types of colonies may be observed on solid media. The first has a flat, grayish appearance with an irregular edge, and a tendency to spread along the direction of the streak, and to swarm and coalesce. The second is convex with an entire edge and a dark center. Most strains are weakly hemolytic on blood agar, but this characteristic may be affected by the composition and pH of the base medium, the composition of the atmosphere, and the time and temperature of incubation. All strains grow in the presence of 1% ox-bile (Vandamme et al., 2005b).
According to ICMSF (1996) the growth temperature range of C. jejuni subsp. jejuni is from 32 to 45°C and the subspecies does not grow at 25 or 47°C. It is not heat-resistant and is easily destroyed by pasteurization, with a D55 value between 0.6 and 2.3 min. The optimal pH is from 6.5 to 7.5, and the maximum pH lies between 9.0 and 9.5. C. jejuni subsp. jejuni does not grow at pH 4.7 and dies rapidly at pH 4.0. It is quite sensitive to drying and does not grow in the presence of 2% NaCl. According to Vandamme et al. (2005b) the strains grow on solid media containing 1–1.5% ox-bile and 0.02% safranin.
Reduction and tolerance of 0.04% triphenyl-tetrazolium chloride is observed in 90% of strains. Most (90–95% respectively) strains grow in the presence of 100 mg/l of 5-fluorouracil and 32 mg/l pf cephalotin.
C. coli cells are small, tightly coiled spiral or S-shaped and transform rapidly to coccoid forms with age or exposure to toxic concentration of oxigen. Colonies on solid media are round, 1–2 mm in diameter, raised, convex, smooth and glistening. On moist media, colonies are flat, grayish, and spread in the direction of the streak. Most but not all strains are non-hemolytic. The strains grow on solid media containing 1–1.5% oxbile, 0.02% safranin, 32 mg/l of cephalotin, and 0.04% triphenyltetrazolium chloride. Reduction of the latter substrate is also observed. Most (approximately 76%) strains are resistant to 100 mg/l of 5-fluorouracil (Vandamme et al., 2005b).
Differentiating C. coli from C. jejuni subsp. jejuni is difficult. The most biochemical test used for this purpose is hippurate hydrolysis, for which C. coli is negative. However, some strains of C. jejuni subsp. jejuni also give a negative result (Vandamme et al., 2005b).
3. Epidemiology
According to WHO (2000) the campylobacteriosis caused by Campylobacter usually has an incubation period of two to five days (can range from one to ten days) and the duration is usually three to six days. The main symptom are diarrhea (frequently containing blood), abdominal pain, fever, headache, nausea, and/or vomiting. These infections are for the most part self-limited and do not require treatment with antibiotics. Complications such as bacteraemia, hepatitis, pancreatitis, and abortion have been reported with different frequency. Post-infection complications may also occur including reactive arthritis and neurological disorders such as Guillain-Barré syndrome (a polio-like form of paralysis that can result in respiratory and severe neurological dysfunction or death in a small number of cases). Death is rare in healthy individuals but can occur in very young or elderly patients, AIDS patients or in the otherwise debilitated.
Campylobacter species are widely distributed among warm-blooded animals and are prevalent in poultry, cattle, swine, sheep, ostriches and shellfish, and in pets including cats and dogs. The primary route of trans-mission is through the consumption of contaminated foods, particularly undercooked meat, meat products and raw milk. Non-treated water is also a recognized source of infection. Campylobacteriosis is considered a direct zoonosis, that is, a disease transmitted from animals or animal products to man. In animals, Campylobacter rarely causes disease (WHO, 2000).
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Gupta, A., Nelson, J.M., Barrett, T.J. Tauxe R.V., Rossiter, S.P., Friedman, C.R., Joyce, K.W., Smith, K.E., Jones, T.F., Hawkins, M.A., Shiferaw, B., Beebe, J.L., Vugia, D.J., Rabatsky-Ehr, T., Benson, J.A., Root, T.P., Angulo, F.J. & NARMS Working Group (2004) Antimicrobial resistance among Campylobacter strains, United States, 1997–2001. Emerging Infectious Diseases, 10(6):1102–1109.
WHO (World Health Organization). (2000) Campylobacter Fact Sheet No 255. [Online] Available from: http://www.who.int/mediacentre/factsheets/fs255/en/ [Accessed 1st November 2011].
Stanley, K. & Jones, K. (2003) Cattle and sheep farms as reservoir of Campylobacter. Journal of Applied Microbiology, 94, 104S–113S.
Tauxe, R.V., Hargrett-Bean, N. & Patton, C.M. (1998) Campylobacter isolates in the United States, 1982–1986. Morbidity and Mortality Weekly Report, 37(SS-2), 1–13.
Vandamme, P., Dewhirst, F.E., Paster, B.J., On, S.L.W. (2005a) Family I. Campylobacteraceae. In: Brenner, D.J., Krieg, N.R. & Staley, J.T. (eds), Bergey’s Manual of Systematic Bacteriology. 2nd edition. Volume 2, Part C. New York, Springer. pp. 1145–1146.
Vandamme, P., Dewhirst, F.E., Paster, B.J. & On, S.L.W (2005b) Genus I. Campylobacter. In: Brenner, D.J., Krieg, N.R. & Staley, J.T. (eds), Bergey’s Manual of Systematic Bacteriology. 2nd edition. Volume 2, Part C. Springer, New York, 2005. pp. 1147–1160.
ICMSF (International Commission on Microbiological Specifications for Foods) (2002) Microorganisms in Foods 7. Microbiological Testing in Food Safety Management. New York, Kluwer Academic/Plenum Publishers.
Pearson, A.D. & Healing, T.D. (1992) The surveillance and control of campylobacter infection. Communicable Disease Review, 2(12), R133-R139.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|