


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 8-3-2016
Date: 27-12-2020
Date: 15-11-2020
|
QUANTUM NATURE OF LIGHT
One of the earliest views of what light is was provided by Isaac Newton early in the eighteenth century. Based on the behavior of light in exhibiting reflection and refraction, he postulated that light was a stream of particles. Although this explanation works well for basic optical phenomena, it fails to explain interference. Later experiments, such as Young’s dual-slit experiment, showed that light did indeed have a wavelength and that such behavior could only be explained by using wave mechanics, a concept Newton had argued against a century earlier. By the end of the nineteenth century, wave theory was well accepted but a few glitches remained: namely, the blackbody spectrum of light emitted from heated objects and issues such as the UV catastrophe, upon which classical physics failed. To explain this, Max Planck (the “father” of quantum mechanics) used particle theory once again. He postulated that the atoms in a blackbody acted as tiny harmonic oscillators, each of which had a fundamental quantized energy that obeyed the relationship
E = hv (1.1)
where h is Planck’s constant and v is the frequency of the radiation emitted. This important equation may also be expressed in terms of wavelength as
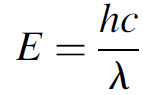 (1.2)
(1.2)
where λ is the wavelength in meters. The ramifications of quantization the fact that the energy of each atom was in integral multiples of this quantity has far reaching ramifications for the entire field of physics (and chemistry), and as we shall see in subsequent chapters, affects our entire view of the atom! Einstein also endorsed this concept of quantization and used it to explain the photoelectric effect, which definitely showed light to exhibit particle properties. These particles of light came to be known as photons, and according to the relationship above, were shown to have a discrete value of energy and frequency (and hence, wavelength). Light can be thought of as a wave that has particle like qualities (or, if you prefer, a particle with wavelike qualities). It was evident from numerous experiments that both wave and particle properties are required to fully explain the behavior of light.
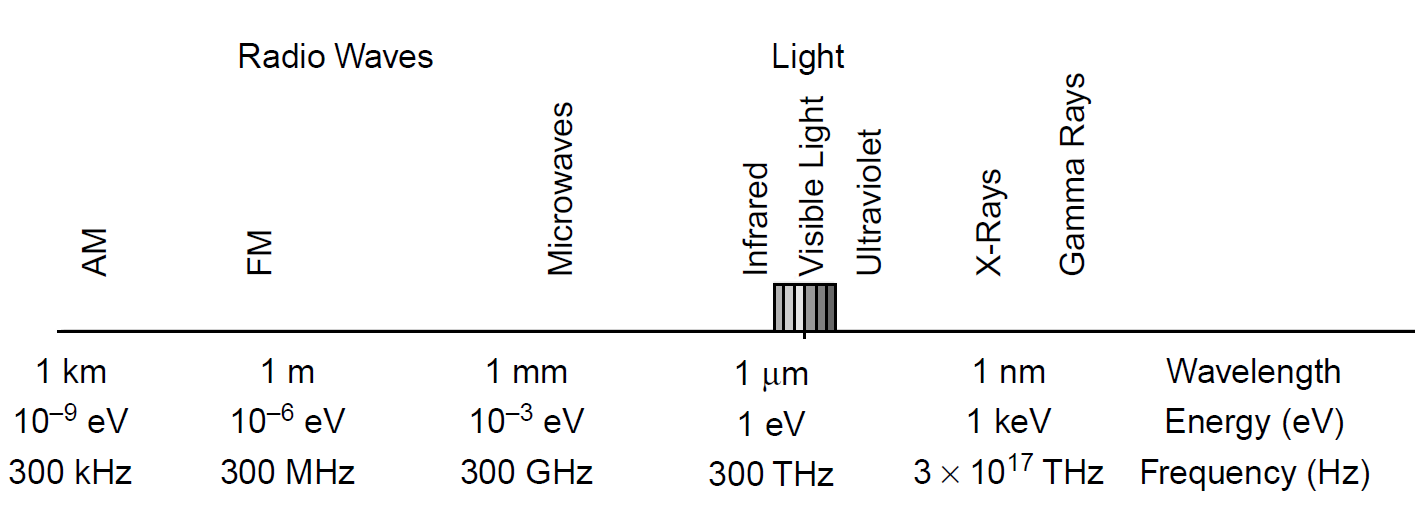
Figure 1.1. Electromagnetic spectrum with energies.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|