


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-2-2016
Date: 22-2-2016
Date: 12-2-2016
|
AMINATION
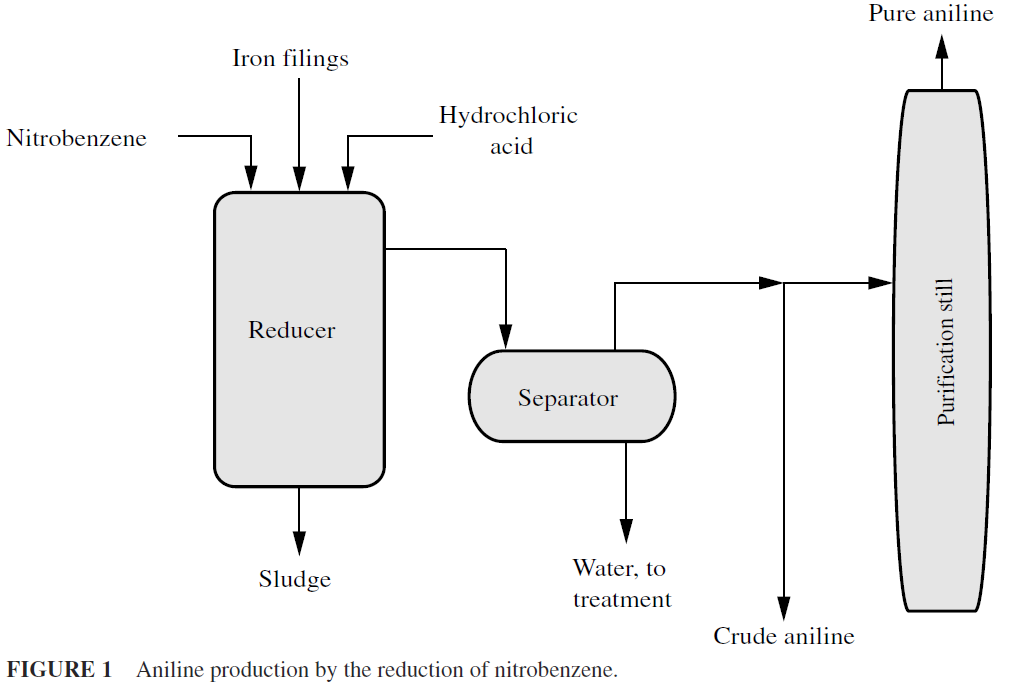
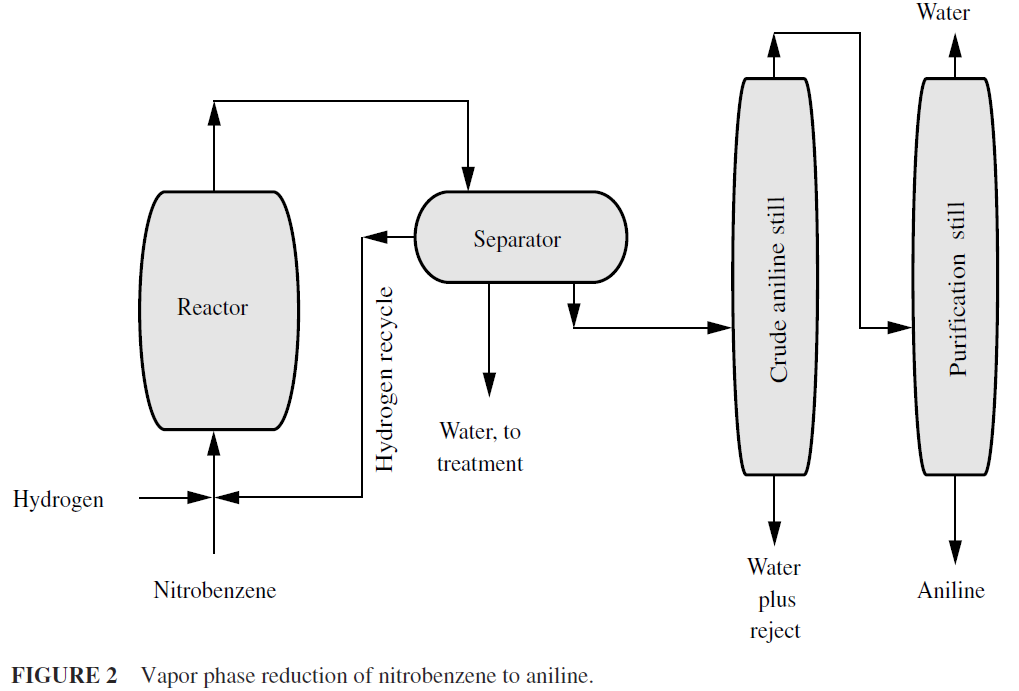
Amination is the process of introducing the amino group (–NH2) into an organic compound as, for example, the production of aniline (C6H5NH2) by the reduction of nitrobenzene (C6H5NO2) in the liquid phase (Fig. 1) or in the vapor phase in a fluidized bed reactor (Fig. 2). For many decades, the only method of putting an amino group on an aryl nucleus involved adding a nitro (–NO2) group, then reduction to the amino (–NH2) group. Without high-pressure vessels and catalysts, reduction had to be done by reagents that would function under atmospheric pressure.
The common reducing agents available under these restrictions are:
1. Iron and acid
2. Zinc and alkali
3. Sodium sulfide or polysulfide
4. Sodium hydrosulfite
5. Electrolytic hydrogen
6. Metal hydrides
Now liquid- and gas-phase hydrogenations can be performed on a variety
of materials.
RNO2 + 3H2 → RNH2 + 2H2O
Where metals are used to produce the reducing hydrogen, several difficult processing problems are created. The expense is so great that it is necessary to find some use for the reacted material. Spent iron can sometimes be used for pigment preparations or to absorb hydrogen sulfide. Stirring a vessel containing much metal is quite difficult.
On a small scale, cracking ammonia can produce hydrogen for reduction. Transport and storage of hydrogen as ammonia is compact, and the cracking procedure involves only a hot pipe packed with catalyst and immersed in a molten salt bath.
The nitrogen that accompanies the generated hydrogen is inert.
Amination is also achieved by the use of ammonia (NH3), in a process referred to as ammonolysis. An example is the production of aniline (C6H5NH2) from chlorobenzene (C6H5Cl) with ammonia (NH3). The reaction proceeds only under high pressure.
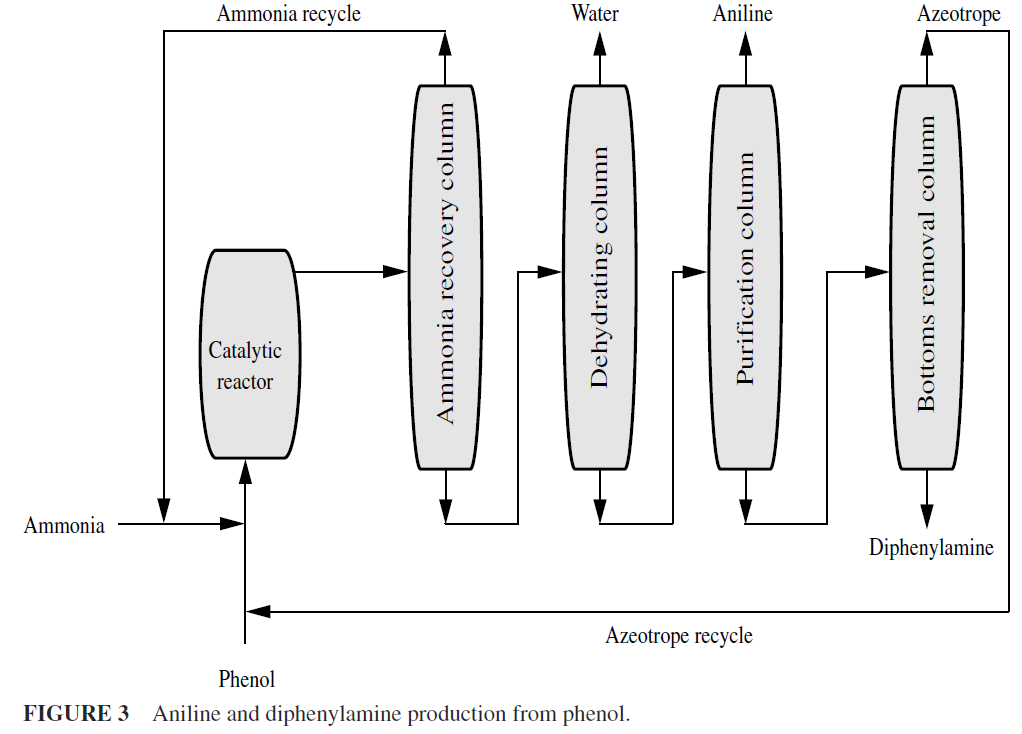
The replacement of a nuclear substituent such as hydroxyl (–OH), chloro, (–Cl), or sulfonic acid (–SO3H) with amino (–NH2) by the use of ammonia (ammonolysis) has been practiced for some time with feedstocks that have reaction-inducing groups present thereby making replacement easier. For example, 1,4-dichloro-2-nitrobenzene can be changed readily to 4-chloro-2-nitroaniline by treatment with aqueous ammonia. Other molecules offer more processing difficulty, and pressure vessels are required for the production of aniline from chlorobenzene or from phenol (Fig. 3).
C6H5OH + NH3 → C6H5NH2 + H2O
Ammonia is a comparatively low cost reagent, and the process can be balanced to produce the desired amine. The other routes to amines through reduction use expensive reagents (iron, Fe, zinc, Zn, or hydrogen, H2, gas) that make ammonolysis costs quite attractive. Substituted amines can be produced by using substituted ammonia (amines) in place of simple ammonia. The equipment is an agitated iron pressure vessel; stainless steel is also used for vessel construction.
Amination by reduction is usually carried out in cast-iron vessels (1600 gallons capacity, or higher) and alkali reductions in carbon steel vessels of desired sizes. The vessel is usually equipped with a nozzle at the base so that the iron oxide sludge or entire charge may be run out upon completion of the reaction. In some reducers, a vertical shaft carries a set of cast-iron stirrers to keep the iron particles in suspension in the lower part of the vessel and to maintain all the components of the reaction in intimate contact. In addition, the stirrer assists in the diffusion of the amino compound away from the surface of the metal and thereby makes possible a more extensive contact between nitro body and catalytic surface.
Thus, amination, or reaction with ammonia, is used to form both aliphatic and aromatic amines. Reduction of nitro compounds is the traditional process for producing amines, but ammonia or substituted ammonias (amines) react directly to form amines. The production of aniline by amination now exceeds that produced by reduction (of nitrobenzene). Oxygen-function compounds also may be subjected to ammonolysis,
for example:
1. Methanol plus aluminum phosphate catalyst yields monomethylamine (CH3NH2), dimethylamine [(CH3)2NH], and trimethylamine [(CH3)3N]
2. 2-naphthol plus sodium ammonium sulfite (NaNH3SO3) catalyst (Bucherer reaction) yields 2-naphthylamine
3. Ethylene oxide yields monoethanolamine (HOCH2CH2NH2), diethanolamine [(HOCH2CH2)2NH)], and triethanolamine
[(HOCH2CH2)3N)]
4. Glucose plus nickel catalyst yields glucamine.
5. Cyclohexanone plus nickel catalyst yields cyclohexylamine Methylamines are produced by reacting gaseous methanol with a catalyst at 350 to 400oC and 290 psi (2.0 MPa), then distilling the reaction mixture.
Any ratio of mono-, di-, or trimethylamines is possible by recycling the unwanted products.
An equilibrium mixture of the three ethanolamines is produced when ethylene oxide is bubbled through 28% aqueous ammonia at 30 to 40 oC.
By recirculating the products of the reaction, altering the temperatures, pressures, and the ratio of ammonia to ethylene oxide, but always having an excess of ammonia, it is possible to make the desired amine predominate. Diluent gas also alters the product ratio.
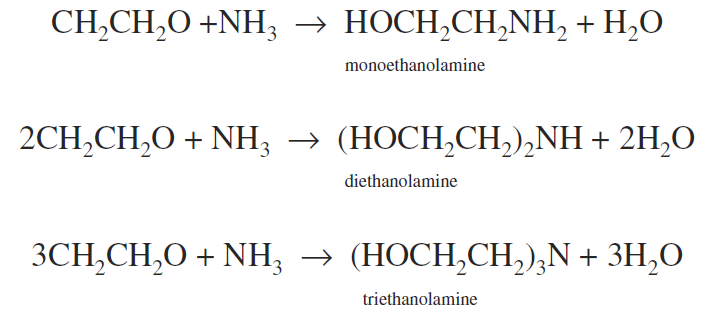
After the strongly exothermic reaction, the reaction products are recovered and separated by flashing off and recycling the ammonia, and then fractionating the amine products.
Monomethylamine is used in explosives, insecticides, and surfactants. Dimethylamine is used for the manufacture of dimethylformamide and acetamide, pesticides, and water treatment. Trimethylamine is used to form choline chloride and to make biocides and slimicides.
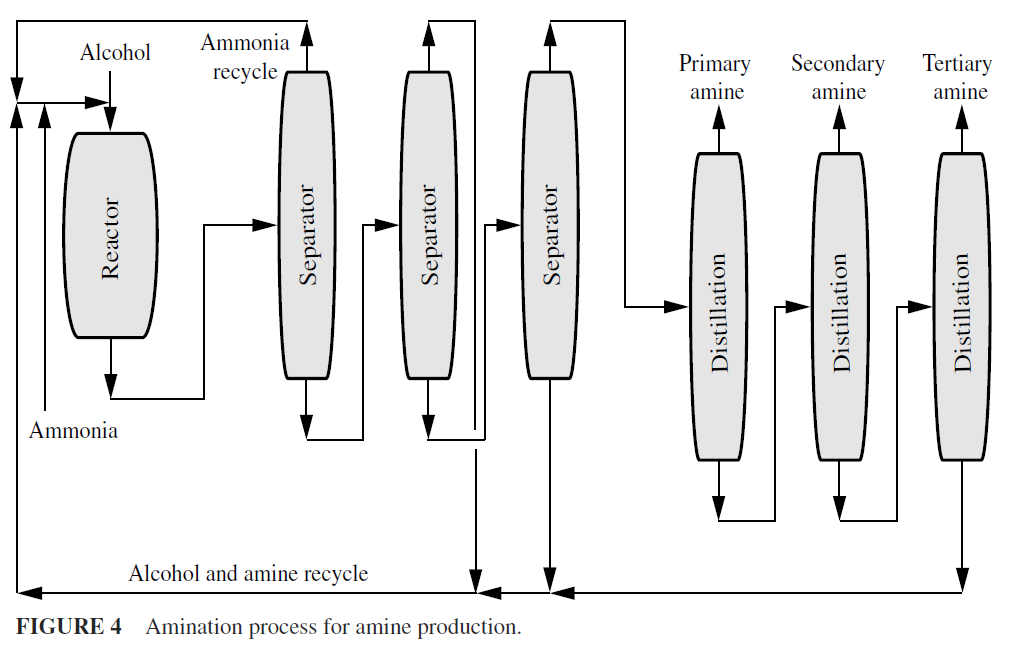
Other alkylamines can be made in similar fashion from the alcohol and ammonia (Fig. 4). Methyl, ethyl, isopropyl, cyclohexyl, and combination amines have comparatively small markets and are usually made by reacting the correct alcohol with anhydrous ammonia in the vapor phase.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
قسم رعاية الصحن ينهي أعماله الخاصة بإقامة الحفل المركزي لتخرج طلبة الجامعات العراقية
|
|
|