


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-8-2017
Date: 8-8-2017
Date: 22-8-2017
|
COAL, OIL SHALE, TAR SAND, AND GAS HYDRATES
Coal, oil shale, and tar sand are carbonaceous materials that can serve as future energy and chemical sources when oil and gas are consumed. The H/C ratio of these materials is lower than in most crude oils. As solids or semi-solids, they are not easy to handle or to use as fuels, compared to crude oils. In addition, most of these materials have high sulfur and/or nitrogen contents, which require extensive processing. Changing these materials into hydrocarbon liquids or gaseous fuels is possible but expensive. The following briefly discusses these alternative energy and chemical sources.
COAL
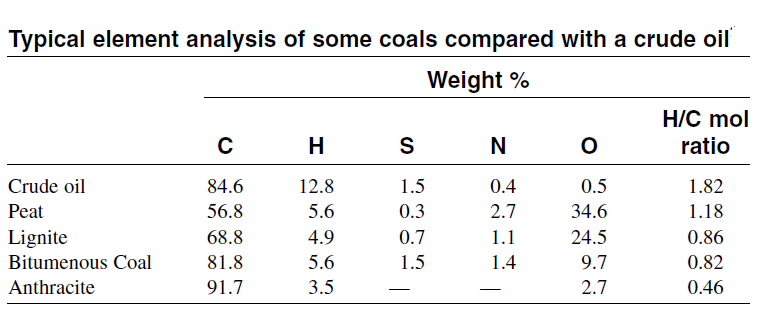
Coal is a natural combustible rock composed of an organic heterogeneous substance contaminated with variable amounts of inorganic compounds. Most coal reserves are concentrated in North America, Europe, and China. Coal is classified into different ranks according to the degree of chemical change that occurred during the decomposition of plant remains in the prehistoric period. In general, coals with a high heating value and a high fixed carbon content are considered to have been subjected to more severe changes than those with lower heating values and fixed carbon contents. For example, peat, which is considered a young coal, has a low fixed carbon content and a low heating value. Important coal ranks are anthracite (which has been subjected to the most chemical change and is mostly carbon), bituminous coal, sub-bituminous coal, and lignite. Table below compares the analysis of some coals with crude oil.
During the late seventies and early eighties, when oil prices rose after the 1973 war, extensive research was done to change coal to liquid hydrocarbons. However, coal-derived hydrocarbons were more expensive than crude oils. Another way to use coal is through gasification to a fuel gas mixture of CO and H2 (medium Btu gas). This gas mixture could be used as a fuel or as a synthesis gas mixture for the production of fuels and chemicals via a Fischer Tropsch synthesis route. This process is operative in South Africa for the production of hydrocarbon fuels.
OIL SHALE
Oil shale is a low-permeable rock made of inorganic material interspersed with a high-molecular weight organic substance called “Kerogen.” Heating the shale rock produces an oily substance with a complex structure.
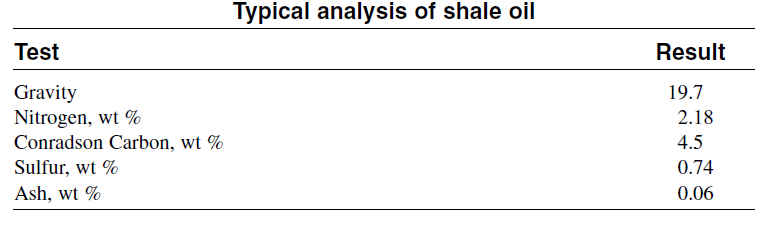
The composition of oil shales differs greatly from one shale to another. For example, the amount of oil obtained from one ton of eastern U.S. shale deposit is only 10 gallons, compared to 30 gallons from western U.S. shale deposits. Retorting is a process used to convert the shale to a high molecularweight oily material. In this process, crushed shale is heated to high temperatures to pyrolyze Kerogen. The product oil is a viscous, highmolecular weight material. Further processing is required to change the oil into a liquid fuel. Major obstacles to large-scale production are the disposal of the spent shale and the vast earth-moving operations. Table below is a typical analysis of a raw shale oil produced from retorting oil shale.
TAR SAND
Tar sands (oil sands) are large deposits of sand saturated with bitumen and water. Tar sand deposits are commonly found at or near the earth’s surface entrapped in large sedimentary basins. Large accumulations of tar sand deposits are few. About 98% of all world tar sand is found in seven large tar deposits. The oil sands resources in Western Canada sedimentary basin is the largest in the world. In 1997, it produced 99% of Canada’s crude oil. It is estimated to hold 1.7–2.5 trillon barrels of bitumen in place. This makes it one of the largest hydrocarbon deposits in the world.24 Tar sand deposits are covered by a semifloating mass of partially decayed vegetation approximately 6 meters thick.
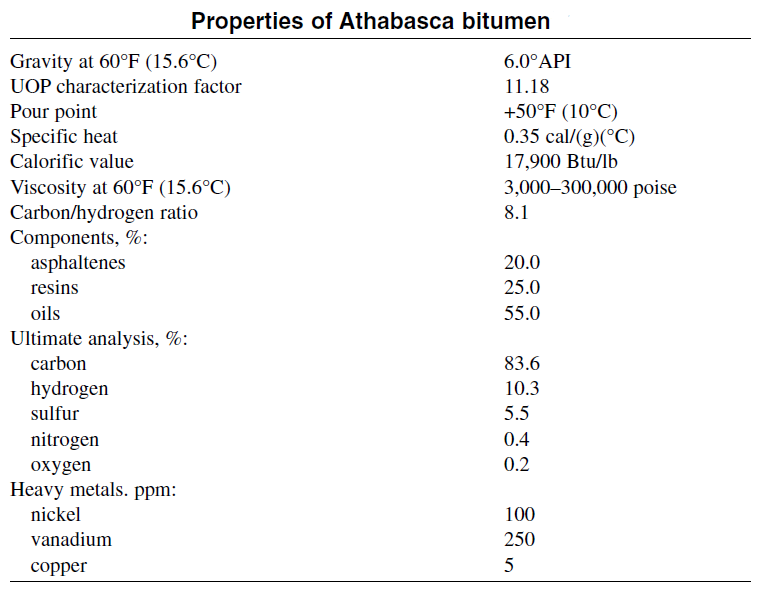
Tar sand is difficult to handle. During summer, it is soft and sticky, and during the winter it changes to a hard, solid material. Recovering the bitumen is not easy, and the deposits are either stripmined if they are near the surface, or recovered in situ if they are in deeper beds. The bitumen could be extracted by using hot water and steam and adding some alkali to disperse it. The produced bitumen is a very thick material having a density of approximately 1.05 g/cm3. It is then subjected to a cracking process to produce distillate fuels and coke. The distillates are hydrotreated to saturate olefinic components. Table below is a typical analysis of Athabasca bitumen.
GAS HYDRATES
Gas hydrates are an ice-like material which is constituted of methane molecules encaged in a cluster of water molecules and held together by hydrogen bonds. This material occurs in large underground deposits found beneath the ocean floor on continental margins and in places north of the arctic circle such as Siberia. It is estimated that gas hydrate deposits contain twice as much carbon as all other fossil fuels on earth. This source, if proven feasible for recovery, could be a future energy as well as chemical source for petrochemicals. Due to its physical nature (a solid material only under high pressure and low temperature), it cannot be processed by conventional methods used for natural gas and crude oils. One approach is by dissociating this cluster into methane and water by injecting a warmer fluid such as sea water. Another approach is by drilling into the deposit. This reduces the pressure and frees methane from water. However, the environmental effects of such drilling must still be evaluated



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|