


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 6-5-2021
Date: 31-12-2015
Date: 2-5-2016
|
Cadherins
The cadherins are a family of transmembrane proteins that regulate cell–cell adhesion in a Ca2+-dependent manner during embryogenesis and as the animal matures (1). Three general groups of cadherins exist within the superfamily: (i) the “classical” cadherins, (ii) the desmosomal cadherins, and (iii) the protocadherins (1). The classical cadherins, of which there are more than 40 examples, display much conservation in their structures. The sequences of their cytoplasmic domains may be as much as 90% identical for proteins within the same species and up to 60% between widely divergent species. In contrast, there is more variation in sequence within the extracellular domains, and 30% to 60% identity is common. These cadherins are located at the adherens junction and at other points of contact between cells that lack a highly organized structure. The desmosomal cadherins, on the other hand, are the Ca2+-dependent cell adhesion molecules found in desmosomes. Within the desmosomal cadherins there are two subclasses: the desmogleins and the desmocollins. Isoforms of both have been characterized. The desmogleins and desmocollins are structurally distinct, implying different roles in vivo. The third group of cadherins, the protocadherins, are more variable in their structures, and their claim for inclusion in the cadherin family lies with the repeating structures in their extracellular domains that are similar to those displayed by the classical and desmosomal cadherins. The protocadherins also have cell adhesion properties, which again link them to the cadherin family.
Cadherins are classified as Type I integral membrane glycoproteins (ie, their N-termini lie on the extracellular side of the cell membrane) and, with one exception (glycosylphosphatidylinositol-anchored T-cadherin), each molecule contains a single a-helical transmembrane-spanning region )M) separating the cytoplasmic from the extracellular domain (1). The extracellular domain generally contains four quasi-repeating motifs (E1, E2, E3, and E4), each about 112 residues long and with one or two potential Ca2+-binding sites per repeat (Fig. 1). A (partial) fifth domain of quite variable length has been designated the extracellular anchor (EA). Some at least of the first four repeats participate in an antiparallel overlap with similar domains in cadherin molecules emanating from other cells, thus facilitating homotypic assembly under Ca2+ control. Other proteins are also believed to be involved in this interaction. Details of the conformation of a cadherin repeat have recently been obtained using nuclear magnetic resonance (NMR) and X-ray diffraction methods (2( 3, .These repeats are also important in determining the specificity of adhesion between the different cadherins. The cytoplasmic domain, which is able to bind a variety of proteins, contains a substructure that shows considerable variations in size between different members of the cadherin family. Consider, for example, human desmoglein and human N-cadherin (4). Both display a proline-rich region (albeit of different lengths) termed the intracellular anchor (IA), followed by a highly charged region (C1). This represents the full extent of the human N-cadherin sequence, in contrast to human desmoglein, which contains a further 320 residues. The C1 domain, being highly conserved between N-cadherin and desmoglein, is likely to be involved in interactions with cytoskeletal microfilaments and other key proteins in the cytoplasm, such as plakoglobin. The remaining part of human desmoglein can be subdivided into a 59-residue proline-rich domain (C2), a region with novel 29-residue repeats (C3) that contain a high density of putative phosphorylation sites, and a C-terminal domain (C4) that is quite divergent in sequence between human and cow, for example. The first half of domain C4 (known as C4a) is very nonpolar and contains two partial repeats of length 28 residues that are rich in glycine residues. They are not related to the 29-residue repeat seen in domain C3. Subdomain C4b is clearly basic in character, in contrast to all of the other cytoplasmic domains, which are acidic. A model of the spatial arrangement of desmoglein in the desmosome has been proposed (4).
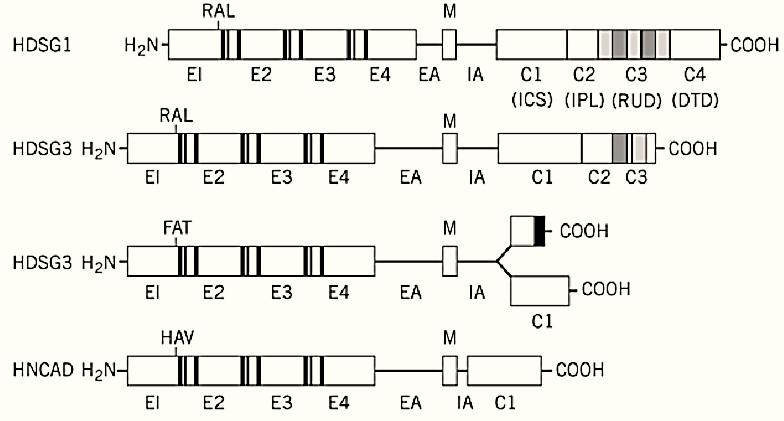
Figure 1. Comparison of the domain structures of human desmoglein 1 (HDSG1), desmoglein 3 (HDSG3), desmocollin 3 (HDSC3), and N-cadherin (NCAD). This illustrates the structural similarities between the classical cadherins and those in the desmosomes. The transmembrane a-helical segment (M) separates the extracellular domains E1 to E4 and the extracellular anchor EA from the intracellular anchor IA and the intracellular domains C1 to C4. Putative calcium-binding domains in the E domains are shown as shaded rectangles. The two desmocollin 3 forms result from alternative splicing. The black rectangle represents 11 amino acids at the C-terminal end that are unique to the shorter structure. (From Ref. 5, with permission.)
References
1. J. A. Marrs and W. J. Nelson (1996) Cadherin cell adhesion molecules in differentiation and embryogenesis. Int. Rev. Cytol. 165, 159–205.
2. M. Overduin, T. S. Harvey, S. Bagby, K. I. Tong, P. Yau, M. Takeichi, and M. Ikura (1995(Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science 267, 386–389.
3. L. Shapiro, A. M. Fannon, P. D. Kwong, A. Thompson, M. S. Lehmann, G. Grubel, J.-F. Legrand, J. Als-Nielsen, D. R. Colman, and W. A. Hendrickson, (1995) Structural basis of cell-cell adhesion by cadherins. Nature (London) 374, 327–337.
4. L. A. Nilles, D. A. D. Parry, E. E. Powers, B. D. Angst, R. M. Wagner, and K. J. Green (1991(Structural analysis and expression of human desmoglein: a cadherin-like component of the desmosome. J. Cell Sci. 99, 809–821.
5. A. P. Kowalczyk, T. S. Stappenbeck, D. A. D. Parry, H. L. Palka, M. L. A. Virata, E. A. Bornslaeger, L. A. Nilles, and K. J. Green (1994) Structure and function of desmosomal transmembrane core and plaque molecules. Biophys. Chem. 50, 97–112.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|