


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 31-12-2015
Date: 9-12-2020
Date: 28-12-2015
|
BAL 31 Nuclease
The enzymes commonly referred to as BAL 31 nuclease have been widely used for manipulation of nucleic acids, most frequently through their ability to shorten both strands of double-stranded DNA from the ends in a controllable manner. Other activities include endonucleolytic attack on double-stranded DNA in response to the presence of a variety of covalent and noncovalent alterations in DNA structure (eg, DNA-carcinogen adducts and junctions between right- and left-handed helical regions) and highly processive unidirectional exonucleolytic degradation of single-stranded DNA.
1.Source and Some Physical Properties
BAL 31 is the strain designation given the original isolates of the marine bacterium that produces the nucleases, which was originally determined to be a species of Pseudomonas (1) but was reclassified as Alteromonas espejiana (2). The nuclease activity was discovered adventitiously as a contaminant in preparations of the Alteromonas bacteriophage PM2, but these enzymes proved to be bacterial products secreted into the culture medium (3, 4). The common name also appears as BAL31 and BAL-31; all should be used when searching electronic databases.
The bulk of the nuclease activity in culture supernatants is owing to two molecularly and kinetically distinct forms, both of which are active as single polypeptide chains (5). The smaller of these, designated the “slow” (S) form, is produced by proteolysis of the larger, “fast” (F) form, which in turn derives from an even larger precursor that has not been characterized (6). Alteromonas espejiana copiously produces extracellular proteinase activity (6), which accounts for the progressive conversion of larger to smaller species in the culture fluid as growth proceeds. At least the S species is not a fully homogenous protein, because its N-terminal amino acid is not unique; either the presumed endoproteolytic event that generates this species from the F form does not occur at a unique site or there is exoproteolytic activity as well. Conversion of the F form to a species indistinguishable in molecular size and catalytic properties from the S form can be done by proteolysis in vitro (6.(
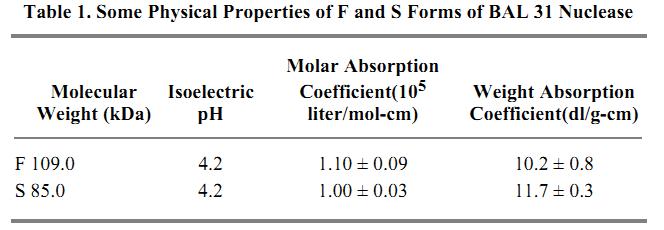
The American Type Culture Collection strain of this organism (ATCC 29659) is suitable for production of the nucleases. No overproducing strains, either of Alteromonas itself or of heterologous hosts containing cloned nuclease genes, have been reported. Purification procedures that effect separation of the S and F forms have been described (5) and modified (6). BAL 31 nuclease is available commercially from several sources, but these products are mixtures of the S and F species. A partial amino acid sequence of an internal fragment produced by cleavage with cyanogen bromide has been obtained (7). This new sequence did not have significant homology with any reported sequences. Some physical properties of the S and F forms are presented in Table 1 (5).
2.Reactions Catalyzed
Five activities appear to account for the degradation—ultimately to mononucleotides—of DNA: (1( a 5′ → 3′ exonuclease activity that acts on single-stranded DNA in a highly processive manner (ie, many nucleotides are removed in a single productive enzyme-substrate encounter) (8); (2) a 3′ → 5′ directed exonuclease activity that acts only on duplex structures, leaving a 5′-terminated single-stranded “tail” (9); (3) an endonuclease activity against single-stranded DNA, (much slower than the exonuclease activity on single-stranded DNA [8]), that is also elicited by a variety of covalent or noncovalent distortions or lesions in duplex DNA (3-5, 10-14); (4) an exonuclease activity that can excise a small number of nucleotides starting from the site of a strand break (nick) in duplex DNA (15), which is inferred to be 5′ → 3′-directed; and (5) an activity that can remove short 3′-terminated tails from otherwise duplex DNA. The F and S forms differ greatly in the rates of removal of nucleotides, at given molar concentrations of DNA ends and enzyme, via the 3′ → 5′ exonuclease action on double-stranded DNA, which led to their “fast” and “slow” designations (5). The two forms have comparable kinetic behavior toward single-stranded DNA (5, 8.(
The BAL 31 nucleases also catalyze the terminally directed hydrolysis of double-stranded RNA and degrade RNA that contains nonduplex structure (16). Whether an analog of the endonuclease activity on altered duplex DNA exists has not been determined, except that cleavage probably occurs in response to a strand break in duplex RNA.
The 5′ → 3′ exonuclease activity on single-stranded DNA is highly processive, as the nucleases are able to degrade DNA polymers of nearly 500 nucleotides in length completely without dissociation of enzyme from substrate (8). The much larger (5,400-nucleotide) fX174 DNA did not appear to be degraded processively, however, so there may be limits to the number of nucleotides removed in a single productive enzyme-substrate encounter (5). The values of Vmax and the turnover number (kcat), on the basis of the rates of internucleotide bond hydrolysis, are similar for the 500-nucleotide polymer described above and the minimal DNA substrate, a dinucleotide diphosphate (8). The kinetic data appear to require a facilitated diffusion mechanism for the activity on macromolecular substrates, in which nuclease molecules bind randomly and diffuse along the contour of the chain until a 5′ end is located, at which time catalysis can begin (8). This apparently was the first example of a requirement for facilitated diffusion in an enzymatic reaction.
The 3′ → 5′ exonuclease activity on duplex DNA and the 5′ → 3′ exonuclease activity on single-stranded DNA can account for the progressive reduction in the length of duplex DNA. Above certain nuclease concentrations, short 5′-terminated single-stranded tails from the 3′ → 5′ exonuclease activity are evident in partial digests (9). If 3′-terminated tails are present, they cannot be more than a few nucleotides in length. The 5′ tails have a limiting length, at the higher enzyme concentrations examined, of about 7 nucleotide residues when about 100 nucleotides are removed by the 3′ → 5′ exonuclease activity. Up to 50% of the ends could be joined in DNA Ligase reactions under conditions favoring the joining of fully base-paired ends. This indicates that the 5′ → 3′ exonuclease activity on single-stranded DNA can terminate at the junction between single- and double-stranded regions, to leave fully based-paired ends on a significant fraction of molecules in a partially digested population. DNA polymerase-mediated repair, to render ends with 5′-terminated tails fully base-paired, markedly increased the fraction of ends joinable by DNA ligase, as expected (9.(
As the nuclease concentrations were decreased below those mentioned above, the average length of the single-stranded tails per 100 nucleotides removed increased dramatically, and the ligase-mediated joining of ends in the absence of polymerase-mediated repair became undetectable. This apparent dependence on the concentration of the enzyme of the relative velocities of two exonuclease reactions that it catalyzes has not been explained. The velocity of the overall reaction to shorten DNA, as measured by the hyperchromicity associated with the hydrolysis of internucleotide bonds, is proportional to enzyme concentration, as expected (17). Repair of ends generated at the low nuclease concentrations noted above with DNA polymerase rendered a high percentage of the molecules ligatable (9.(
There must be an activity that can remove short 3′-terminated protruding single-stranded tails from duplex DNA, because the nuclease readily degrades such starting substrates (9). No evidence exists, however, for 3′ terminally directed hydrolysis of single-stranded DNAs (8.(
The value of the Km (Michaelis constant), in terms of the molar concentration of DNA ends, for the overall exonucleolytic shortening of duplex DNA by the S form is so large that it is not practical for the substrate concentration to approach it in actual reactions. Consequently, the enzyme velocity is directly proportional to substrate concentration over the accessible range (18). A value for this Km was reported in earlier work (5), but it appears to have been determined under conditions for which the substrate concentration did not exceed that of the enzyme, violating a premise of Michaelis-Menton-based kinetics. Kinetic parameters (Vmax per unit concentration of nuclease and Km) for the length reduction of duplex DNA by the F form can be measured accurately, because the Km value is much lower than for the S form and lies in the range of experimentally accessible concentrations of DNA ends. More recent values (18) are in reasonable agreement with the earlier data (5.(
These kinetic parameters for the F nuclease depend on the length of the DNA substrate (7) (not determinable for the S form as noted). This has been shown (7) to be consistent with a model in which nonspecific binding of the nuclease away from the ends is followed by a “search” process to form a productive enzyme-substrate complex, with the enzyme bound to a terminus, as for the degradation of single-stranded DNA. However, such a mechanism is not required by the kinetics as in the case of single-stranded DNA (noted in the text above.(
The kinetics of length reduction of duplex DNA are also dependent on its guanine + cytosine(G + C(content (7, 18). This is significant in the case of the S form of the nuclease, for which the rate of nucleotide removal from DNA ends decreases over fourfold over the range of 37–66 mole% G + C residues. This dependence is somewhat less for the F enzyme. Data are available to predict these effects of DNA base composition on the kinetics (18).
In contrast to the high processivity for the 5′ → 3′ exonuclease action on single-stranded DNA noted earlier, the 3′ → 5′ exonuclease is quasi-processive, removing only about 18 and 28 nucleotide residues per productive binding event for the S and F enzymes, respectively (9).
The length reduction of duplex RNA (16) presumably proceeds by a similar mechanism but has not been characterized. The BAL 31 nucleases are the most efficacious enzymes known for the controlled length reduction of duplex DNA, and they are apparently the only enzymes that can catalyze this reaction for duplex RNA.
Duplex DNA is not attacked endonucleolytically (away from an end) at a significant rate unless there is some alteration of the duplex structure. Hence, nonsupercoiled, closed circular duplex DNA (form I° DNA) is extremely resistant to attack by the BAL 31 nucleases (11, 13, 14). The very limited attack on form I° DNA at high enzyme concentrations and long incubations (14), plus the kinetic parameters for exonucleolytic degradation of linear duplex DNA, lead to estimates of the relative rates of introduction of endonucleolytic breaks to exonucleolytic scissions of 8 × 10–11 and 7 × 10 -12 for the S and F enzymes, respectively.
Negative supercoiling in closed circular DNA can elicit the endonuclease activity (12). In the majority of molecules of such supercoiled DNA, an endonucleolytic event (cleavage in one strand) is followed by the removal, in a processive manner, of several nucleotides (6.5 and 2.8 nucleotides for the F and S forms, respectively) from the initially nicked strand, to yield a gapped circular DNA intermediate (15). A fraction contain only a strand break, and no nucleotides are excised. The percentage of molecules with no nucleotides removed is significantly lower for the F nuclease than for the S species. The nicks and gaps are bounded by 5′-phosphoryl and 3′-hydroxyl termini. It is assumed that other alterations that result in endonucleolytic attack give rise to such gapped circular intermediates.
The removal of a small number of nucleotides could be accomplished exonucleolytically, starting from the site of the initial nick. Or, there could be a second endonucleolytic cut a few nucleotides away from the first one; the short oligonucleotide between endonucleolytic breaks would dissociate at room temperature to leave a gap. Available evidence supports the exonucleolytic mechanism. It was reasoned that the presumed exonucleolytic activity producing the gaps should be 3′ → 5′–directed, as the exonucleolytic activity attacking base-paired ends has this characteristic. However, this proved to be inconsistent with the results of further experiments carried out assuming the 3′ → 5′ mode of attack; the 5′ → 3′ attack is thus inferred. Because this activity operates on nominally duplex DNA, it might be expected that it could remove nucleotides in a 5′ → 3′ direction from fully based-paired ends, to leave 3′-terminated single-stranded ends. It was noted that, if these are present, they could not be more than a few nucleotides in length, but this does not rule out very limited activity comparable to that producing the gaps.
The nicked and gapped circular DNA intermediates are then converted to linear duplex DNA by a second endonucleolytic event in the other strand, which requires a second encounter with a nuclease molecule (15). The resulting linear duplex DNA is further degraded by the mechanism noted in the text above. As expected, circular duplex DNA containing a nick introduced by means other than BAL 31 nuclease action is converted to linear duplex DNA, with the occurrence of gaps of the average sizes noted above in most of the circular molecules before their linearization (15).
3.Effects of Solvent Variables and Denaturing Agents
Both Ca2+ and Mg2+ at concentrations above 10–12 mM are required for maximum activity on both single- and double-stranded substrates (9). All activities are optimal near neutral pH (4, 9). The nucleases are remarkably resistant to inactivation at elevated salt concentrations (4, 10). The reaction buffer in which most of the work described above was done contained 0.6 M NaCl). This salt tolerance is not surprising, considering that sea water is the natural milieu of these extracellular enzymes. High concentrations of agents that normally denature proteins fail to eliminate the nuclease activities. Single-stranded DNA is degraded at 40% of the maximum rate in 6.5 M urea (4), and substantial activity on this substrate was found in the presence of 6 M guanidinium chloride. Crude preparations maintained activity against both single- and double-stranded substrates in the presence of 5% (w/v) SDS (3). At least a portion of the structure of the S nuclease does not become disrupted under the stringent denaturation conditions used for denaturing SDS-PAGE (6).
The nucleases are not remarkably resistant to thermal inactivation, as the half-life for disappearance of the activity on single-stranded DNA is only 3–5 minutes at 50°C (7). However, tests on preparations stored at 4°C imply that most of the activity should be retained for years in a buffer containing 5 mM Mg2+ and Ca2+ (4). Commercial preparations are often supplied in 50% (v/v(glycerol for storage at –20°C , under which conditions they should retain full activity indefinitely.
4.Applications
By far the most extensive use of the BAL 31 nucleases, represented by hundreds of literature citations, has been the controlled length reduction of linear duplex DNA. The bulk of these reports describe the production of deletion mutants and/or the elucidation of sequences required for a particular biological activity of a (usually) cloned DNA segment. Cloned sequences can be deleted unidirectionally by cleavage of the vector containing the cloned insert at a unique restriction site on one side of the insert, carrying out a partial BAL 31 degradation, releasing the shortened insert from the shortened vector by use of a restriction site on the other side of the insert, and ligation to an intact linearized vector DNA (19.(
Numerous reports have appeared of BAL 31 nuclease-mediated identification of telomeric sequences, which occur at the ends of linear eukaryotic chromosomes and hence are degraded first by the exonuclease in intact DNA; Yao and Yao (20) and De Lange and Borst (21) apparently represent the earliest work. Where these DNAs are too large to isolate as intact molecules in solution, in situ lysis in agarose has been used (22) so that the initial substrate DNA is largely intact.
The progressive removal of sequences from duplex ends allows the determination of the restriction map of a DNA, either naturally linear or linearized at a unique site, by noting the order in which fragments from subsequent digestion with the restriction enzyme in question disappear from gel electrophoresis patterns of progressively shortened aliquots (19, 23). This technique is greatly enhanced if unidirectional deletions can be done, as noted above (19), as ambiguity arising from loss of sequences from both ends of the fragment is eliminated. The sites of bound proteins, including nucleosomes, and interstrand crosslinks that block the exonuclease can be elucidated because intact duplex sequences between such sites will not be attacked (24, 25).
The endonuclease activity that cleaves in response to alterations in duplex structure has been used in several laboratories to detect such alterations. This attack will be followed by cleavage of the other strand and exonucleolytic attack from the ends thus generated, as noted in the text above. Where chemical modification is done, form I° DNA is used (this requires that the modification does not introduce nicks), and the rate of its loss on incubation with nuclease is monitored. Some of the chemical lesions that give rise to endonucleolytic attack are pyrimidine dimers and possibly other photoproducts of ultraviolet irradiation, adducts with carcinogens such as N-acetoxy-N-2-acetylaminofluorene and N-methyl-N-nitrosourea, interstrand cross-links produced by reaction with nitrous acid, apurinic sites, and adducts with Hg2+ and Ag+ ions (11, 13, 14). Strand breaks were noted in the text above as eliciting cleavage of the opposite strand. Noncovalent alterations eliciting endonucleolytic attack include moderate degrees of negative supercoiling and very high degrees of positive supercoiling (12), junctions between right-handed B-DNA and left-handed Z-DNA regions (10), the presence of unpaired nucleotides in one strand of an otherwise duplex DNA (26), and cruciform structures resulting from the extrusion of inverted repeated sequences under supercoiling stress (27, 28). Matrix attachment sites for nucleolar DNA are apparently sensitive to the nuclease (29). Finally, the lack of sensitivity of form I° DNAs has been used to help identify such species in DNA populations (30).
References
1.R. T. Espejo and E. S. Canelo (1968) J. Bacteriol. 95, 1887–1891.
2.K. Y. Chan, L. Baumann, M. M. Garza, and P. Baumann (1978) J. Syst. Bacteriol. 28, 217–222.
3.H. B. Gray, Jr., D. A. Ostrander, J. L. Hodnett, R. J. Legerski, and D. L. Robberson (1975(Nucleic Acids Res. 2, 1459–1492.
4.H. B. Gray, Jr., T. P. Winston, J. L. Hodnett, R. J. Legerski, D. W. Nees, C.-F. Wei, and D. L. Robberson (1981) In Gene Amplification and Analysis (J. G. Chirikjian and T. S. Papas, eds.(, Elsevier North-Holland, New York, pp. 169–203.
5.C.-F. Wei, G. A. Alianell, G. H. Bencen, and H. B. Gray, Jr. (1983) J. Biol. Chem. 258, 13506–13512.
6.C. R. Hauser and H. B. Gray, Jr. (1990) Arch. Biochem. Biophys. 276, 451–459.
7. T. Lu (1992) Some physical and catalytic properties of BAL 31 nuclease, Ph.D. dissertation, University of Houston, Houston, Texas.
8.T. Lu and H. B. Gray, Jr. (1995) Biochim. Biophys. Acta 1251, 125–138.
9.X.-G. Zhou and H. B. Gray, Jr. (1990) Biochim. Biophys. Acta 1049, 83–91.
10.M. W. Kilpatrick, C.-F. Wei, H. B. Gray, Jr., and R. D. Wells (1983) Nucleic Acids Res. 11, 3811-3822.
11.R. J. Legerski, H. B. Gray, Jr., and D. L. Robberson (1977) J. Biol. Chem. 252, 8740–8746.
12.P. P. Lau and H. B. Gray, Jr. (1979) Nucleic Acids Res. 6, 331–357.
13.C.-F. Wei, G. A. Alianell, H. B. Gray, Jr., R. J. Legerski, and D. L. Robberson (1983) In DNA Repair: A Laboratory Manual of Research Procedures (E. C. Friedberg and P. C. Hanawalt, eds.), vol. 2, pp. 13–40.
14.C.-F. Wei, R. J. Legerski, G. A. Alianell, D. L. Robberson, and H. B. Gray, Jr. (1984) Biochim. Biophys. Acta 782, 404–414.
15.A. Przykorska, C. R. Hauser, and H. B. Gray, Jr. (1988) Biochim. Biophys. Acta 949, 16–26.
16.G. H. Bencen, C.-F. Wei, D. L. Robberson, and H. B. Gray, Jr. (1984) J. Biol. Chem. 259, 13584-13589.
17.X.-G. Zhou (1989) Ethidium bromide-mediated renaturation of denatured closed circular DNAs in alkaline solution: mechanistic aspects and fractionation of closed circular DNAs on a molecular weight basis. Some catalytic properties and mechanism of exonuclease action of BAL 31 nuclease, Ph.D. dissertation, University of Houston, Houston, Texas.
18.H. B. Gray, Jr. and T. Lu (1993) In Enzymes of Molecular Biology (M. M. Burrell, ed.), Humana Press, Totowa, New Jersey, pp. 231–251.
19.C. R. Hauser and H. B. Gray, Jr. (1991) Gene Anal. Tech. Appl. 8, 139–147.
20.M.-C. Yao and C. H. Yao (1981) Proc. Natl. Acad. Sci U.S.A. 78, 7436–7439.
21.T. De Lange and P. Borst (1982) Nature 299, 451–453.
22.R. F. Wintle, T. G. Nygaard, J. A. Herbrick, K. Kvaloy, and D. W. Cox (1997) Genomics 40, 409-414.
23.R. J. Legerski, J. L. Hodnett, and H. B. Gray, Jr. (1978) Nucleic Acids Res. 5, 1445–1464.
24.W. A. Scott, C. F. Walter, and B. L. Cryer (1984) Mol. Cell. Biol. 4, 604–610.
25.W.-P. Zhen, O. Buchardt, H. Nielsen, and P. E. Nielsen (1986) Biochemistry 25, 6598–6603.
26.D. H. Evans and A. R. Morgan (1982) J. Mol. Biol. 160, 117–122.
27.L. H. Naylor, H. A. Yee, and J. H. van de Sande (1988) J. Biomol. Struct. Dyn. 5, 895–912.
28.N. M. Morales, S. D. Coburn, and U. R. Muller (1990) Nucleic Acids Res. 18, 2777–2782.
29.O. V. Iarovaia, M. A. Lagarkova, and S. V. Razin (1995) Biochemistry 34, 4133–4138.
30.E. Cuzzoni, L. Ferretti, C. Giordani, S. Castiglione, and F. Sala (1990) Mol. Gen. Genet. 222, 58-64.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|