


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 2025-02-22
Date: 19-11-2015
Date: 20-11-2015
|
Paramyxoviruses
This family includes the genera:
-Parmyxovirus with the parainfluenza viruses.
-Rubulavirus with the mumps virus.
-Morbillivirus with the measles virus.
-Pneumovirus with the respiratory syncytial virus (RS).
-Nonclassified paramyxoviruses (Hendra, Nipah).
The clinical manifestations include respiratory infections (parainfluenza, pseudocroup, mumps, RS, Nipah, Hendra), parotitis, and infections of other glandular organs (mumps), exanthem (measles), and CNS infections (mumps, measles, Nipah, Hendra).
Prevention: live vaccines are used to protect against measles and mumps; no immunoprophylactic tools are available against the other paramyxoviruses.
Pathogen. The family Paramyxoviridae is a heterogeneous one, both in its biology and pathogenic properties. It is divided into two subfamilies:
-Paramyxovirinae with the genera:
*Paramyxovirus with the human pathogen species parainfluenza virus types 1 and 3.
*Rubulavirus with the mumps virus and parainfluenza virus types 2 and 4.
*Morbillivirus with the human pathogen measles virus and several zoo- pathic species that cause severe respiratory infections in various animal species (dogs [canine distemper], cats, cattle, seals, dolphins, turtles).
The nonclassified, closely related zoopathic and human pathogen Hendra and Nipah viruses.
-Pneumovirinae, genus Pneumovirus, probably with several types of RS virus (respiratory syncytial virus).
Structure of the Paramyxoviruses
All paramyxoviruses have a similar structure (Fig. 1). They are pleomorphic. The smallest forms are 120-150 nm in size (with the exception of the somewhat smaller RS virus). They also possess an envelope that encloses the nucleocapsid. The genome consists of a continuous, single-stranded antisense RNA. The envelope is derived from the cell membrane. Various viral proteins are integrated in the envelope, visible in the form of spikes. The generic taxons are based on these spikes: parainfluenza and mumps viruses have two types of spikes, one containing the hemagglutinin (i.e., possessing hemagglutination activity) coupled with neuraminidase (HN protein), and the other the so-called fusion (F) protein, responsible for fusion of the envelope with the cell membrane. Measles viruses contain no neuraminidase and pneumoviruses possess only the F protein.
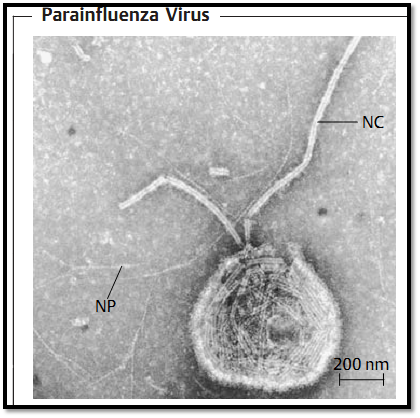
Fig. 1 The envelope here is torn open, allowing the helical nucleocapsid (NC) to escape. NP: primary nucleoprotein helix (TEM).
Pathogenesis and clinical picture
-The parainfluenza viruses cause flulike infections, mainly in small children, which occasionally progress to bronchitis or even pneumonia. Occasionally, a dangerous croup syndrome develops. Bacterial superinfections are frequent, as are the usually harmless reinfections.
-In mumps virus infections the virus first replicates in the respiratory tract, then causes a viremia, after which a parotitis is the main development as well as, fairly frequently, mumps meningitis. Complications include infection of various glandular organs. Orchitis can occur in postpuberty boys who contract mumps.
-Measles. The pathogenesis of measles has not been fully explained. It is assumed that the virus, following primary replication in lymphoid tissues, is distributed hematogenously in two episodes. Thereafter the oral mucosa displays an enanthem and the tiny white “Koplik's spots.” Then the fever once again rises and the typical measles exanthem manifests (Fig. 2). Possible complications include otitis in the form of a bacterial superinfection as well as pneumonia and encephalitis. A rare late sequel of measles (one case per million inhabitants) is subacute sclerosing panencephalitis (SSPE) in which nucleocapsids accummulate in brain cells, whereby few or no viral progeny are produced for lack of matrix protein. This disease occurs between the ages of one and 20, involves loss of memory and personality changes, and usually results in death within six to 12 months.
-Nipah and Hendra virus infections are zoonoses endemic to Southeast Asia (Nipah) or Australia (Hendra). Both infections result in encephalitis with relatively high lethality rates (up to 40%) and in some cases severe interstitial pneumonias.
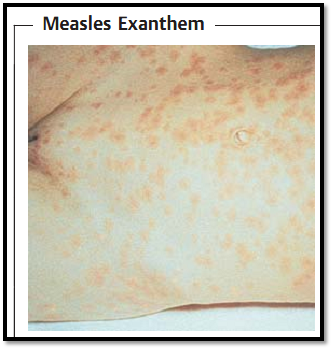
Fig. 2 The typical exanthem manifests during what is presumably the second hematogenous disseminative episode of the morbilliviruses.
-RS viruses cause bronchiolitis or pneumonia, mainly in children up to six months of age, or rarely up to two years. Immune status appears to play an important role in the course of the infection. It has been determined that the course of the disease is more severe in children who have received dead vaccine material (similarly to measles). This is presumably due to antibodies, in the case of small children the mother's antibodies acquired by diaplacental transport. Immunosuppressed patients, for instance, bone marrow recipients, are also at risk for RSV.
Diagnosis. In addition to serodiagnostic methods, direct detection tests based on immunofluorescence or enzyme immunoassay are available for paramyxoviruses, some of them quite sensitive. Paramyxoviruses replicate readily in cell cultures from human tissues.
Epidemiology. Paramyxoviruses are transmitted by droplet infection. Generalized contamination levels in the population (except for Nipah and Hendra) are already very high in childhood (90% in 10-year-old children for parainfluenza virus types 1-3).
Nipah and Hendra viruses are zoonoses that are transmitted to humans from animals (Nipah: pigs, Hendra: horses). Various different animals can be infected by these pathogens, but bats (Pteropus) appear to be the natural reservoir for both viruses.
Prevention. Attenuated live vaccines are available for measles and mumps. The dead vaccine should not be used due to the aggravating effect mentioned above. No vaccines have as yet been developed for the other parainfluenza viruses.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
ملاكات العتبة العباسية المقدسة تُنهي أعمال غسل حرم مرقد أبي الفضل العباس (عليه السلام) وفرشه
|
|
|