


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 10-11-2015
Date: 11-11-2015
Date: 10-11-2015
|
Immunogenetics
INTRODUCTION
The main function of immune system is to identify and attack foreign antigens. Knowledge of genes of the immune system is of clinical importance in studying the response to infection, study of autoimmune disease and transplantation technology. Most of the antigens are proteins, but some are polysaccharides and some nucleic acids. In addition to being the causative factor for a number of single gene disorders, the genes of the immune system provide models for study of gene expression. The genes of the immune system have great diversity in a few loci (Fig. 1).
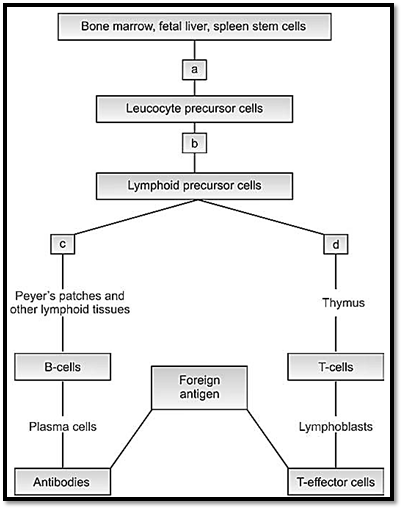
Fig. 1: Sites of blocks in the generation of immunodeficiency syndromes. (a) Reticular dysgenesis (b) severe combined immunodeficiency syndrome (c) Bruton's agamaglobulinemia (d) DiGeorge syndrome.
There are two components to an immune response cellular and humoral. The thymus is the primary lymphoid organ where cells differentiate into thymus dependent or T cells. Lymphoid stem cells are present in the growing fetus. Lymphocytes in the secondary lymphoid organs like the spleen or in the cortical regions of the lymph nodes differentiate into B cells.
Cellular immunity is produced by T lymphocytes, and is responsible for homograft rejection and delayed hypersensitivity. The T lymphocyte cells have two functions, they act as cytotoxic or helper cells. The cytotoxic or killer lymphocytes are sensitized to destroy cell-bearing antigens produced by viral infections. The other group, helper lymphocytes are necessary for induction of antibody response by B-lymphocytes. A third group is called the suppresser lymphocytes, which suppress immune responses.
Humoral immunity is produced by differentiation of lymphocyte stem cells into plasma cells or B-lymphocytes. They are responsible for production of antibodies or immunoglobulins. The differentiation occurs in the fetal liver, spleen and bone marrow. These are the primary lymphoid organ sites where general hematopoiesis occurs. As this initially takes place in the Bursa of Fabricius (fetal equivalent of these tissues), they are known as B cells. Plasma cells are formed in the secondary lymphoid organs, the red pulp of the spleen and medulla of lymphoid nodes.
The T lymphocyte binds with the antigen through its receptor T cell on the surface of the cells, in conjunction with the major histocompatibility complex. A number of lymphokines are released, which promote division of polymorphonuclear leukocytes, macrophages and B-lymphocytes.
Immunoglobulins
Immunoglobulins are antibodies, and form one of the important and major classes of serum proteins responsible for the body’s defense mechanism against infection by its antigenic properties.
Structure of Immunoglobins
In any individual, an infinite number of different antibodies are encoded on the germline DNA. It is estimated that about 108 different antibodies are produced, even though the number of base pairs of DNA is only 3 X 106. This is because a relatively small number of genes in the germline that encode antibodies undergo a process of somatic rearrangement and recombination during B-cell development, which probably allows for the diversity.
The immunoglobulin molecule is made up of four polypeptide chains, two identical heavy chains (H) with 440 amino acids and two identical light chains (L) with 220 amino acids. These are held together in a Y shape by disulphite bonds (Fig. 2). A eolytic enzyme, papaine cuts immunoglobulin into three fragments, two of, which are similar, each containing an antibody site that can combine with a specific antigen. This is called antigen binding fragment or Fab. The third fragment can be
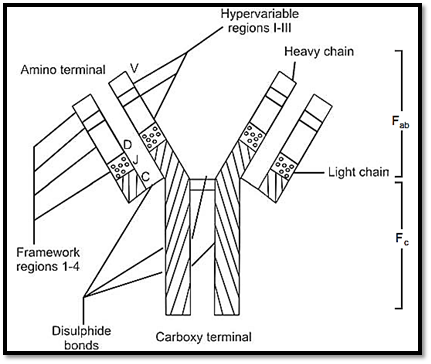
Fig. 2: Structure of an antibody molecule V is the variable region, D is the diversity region, J is the joining region, C is the constant region
crystallized and is called Fc that binds complement and receptors or cell types involved in immune response.
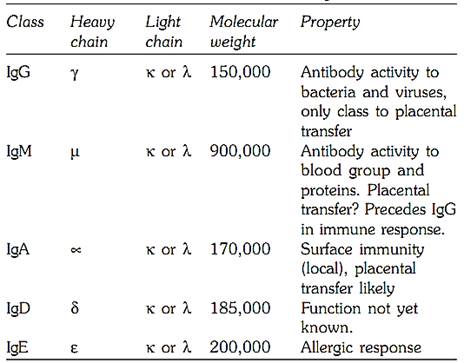
The ‘H’ chains are sub divided into five isotopes, on the basis of structural differences at the carboxyl terminal portion of the H chains. The Fab fragment is composed of L chains and linked with ‘N’ terminal portion of the H chains. The L chains are of two types, kappa (K) or lambda (λ). The heavy chains are of five different classes ϒ, |µ, ∝, δ and Ɛ, and each immunoglobulins IgG, IgM, IgA, IgD, and IgE. All five classes of immunoglobulin have two types of chains, and the molecular formula therefore reads λ 2, or k2r2. Table 13.1 gives outline of these immunoglobulin.
All normal individuals have the five classes of immunoglobulins. Their genetically determined variants have also been recognised. Those associated with the heavy chain of IgG are called Gm systems, and the Am System is associated with IgA heavy chain. Km and Inv systems are associated with kappa light chain. The Oz system is for the λ light chain and the Em for IgE heavy chain. The Gm and Km systems are not interdependent and are polymorphic with frequent variations in different ethnic groups.
Table 13.1: Classes of immunoglobulin
Immunoglobulin Diversity
Various combinations of heavy and light chains are responsible for immunoglobulin (antibody) diversity. Several genes would be required for this. In multiple myleoma, study of Bence Jones proteins revealed immunoglobulins have two regions. Variable region V and constant region C. Region V is further subdivided into four regions. These are further subdivided into three regions, which show great variation, and are called hypervariable region. DNA studies have shown that segments coding for the V and C regions, are separated by a J region, which joins the two. DNA sequencing of heavy chain genes shows that they are coded by four different DNA segments, one each for V, D, C, and J.
Chromosomal Locations of Immunoglobulins
Immunoglobulins have their locations on autosomes 14, 2, and 22. Their synthesis is an exception to the one gene-one polypeptide theory, as V and C regions of each chain are coded differently by different genes.
Inherited immunogenetic disorders
Immunodeficiency disorders can be divided into primary immunodeficiency disorders, which are usually genetically determined and secondary immunodeficiency disorders. Primary immunodeficiency disorders affect specific immunity or nonspecific host defense mechanisms mediated by complement proteins and phagocytosis.
Primary immunodeficiencies are divided into several groups:
1- Deficiency of phagocytic cell function
2- Deficiency of a complement protein
3- Deficiency of B cell development or function
4- Deficiency of T cell development or function
5- Combined B and T cell deficiencies
DEFICIENCY OF PHAGOCYTIC CELL FUNCTION
Another mechanism involved in the body’s defense system is chemotaxis and phagocytosis responsible for cell mediated killing of micro organisms. The disorders seen include Chronic granulomatous disease, leucocyte adhesion deficiency, glucose 6 phospahte dehydrogenase deficiency (which involves a defect in the hexose monophoshate shunt), myeloperoxidase deficiency (a granule enzyme deficiency), and Chediak Higashi syndroms (a granule structural defect which manifests with recurrent infection with bacteria due to chemotactic and degranulation defects). A few of these are discussed below.
Chronic Granulomatous Disease
The molecular defect is deficiency of NADPH oxidase. Chronic granulomatous disease (CGD) is inherited as an X-linked or autosomal recessive disorder. Due to a disorder of phagocytic function patients get recurrent catalase positive bacterial or fungal infections. There is high childhood mortality, unless supportive treatment with prophylactic antibiotics is used. CGD is diagnosed by an abnormal nitroblue tetrazolium test (NBT). The test is based on the increase in metabolic activity of normal granulocytes after phagocytosis, and the absence of such an increase in CGD.
Leucocyte Adhesion Deficiency
Individuals with leucocyte adhesion deficiency have an increased susceptibility to bacterial infections of skin and mucous membranes, which can be life threatening. The cause of increased susceptibility is due to inability of phagocytosed cells to migrate, as a result of adhesion related functions like chemotaxis and phagocytosis, and absence of the B2 integrin receptor. Bone marrow transplant is the treatment of choice; however antibiotics are required prophylactically until this can be done.
Deficiency of a complement protein
The complement system is a complex system of nine distinct serum proteins designated C1 through C9 that require serial activation through the classical or alternative pathways. The interaction of various components of complement often called as Complement Cascade results in increased inflammation and vascular permeability. Patients with complement protein deficiencies are susceptible to different diseases depending on which component is missing, since different complement proteins have different biological functions. Frequent modes of presentation for complement component deficiencies are collagen vascular diseases for C1 through C4, disseminated infections with pyogenic bacteria for C3, and disseminated Neisserial infections for C5 through C8. The most common deficiency is that of the C1 esterase inhibitor of the complement system. This causes hereditary angioneurotic edema, described below.
Angioneurotic Edema
Angioneurotic edema is inherited as an autosomal dominant disorder. This is a severe condition and is characterized by recurrent episodes of edema of the skin, throat or gut which is life threatening and poses a challenge for treatment. The condition occurs due to deficiency of an inhibitor of the first component of complement C1 The deficiency lies either in the total amount of inhibitor, or lack of functional activity in the normal amounts of inhibitor. The treatment consists of infusion with normal fresh-frozen plasma during an acute attack. The androgenic drug Danazol and E-dminocaproic acid can be used to prevent the attacks.
Feficiency of b cell development or function
These include transient neonatal hypogammaglobulinemia, common variable immunodeficiency, selective IgA deficiency, and Bruton’s agammaglobulinemia, discussed below.
Bruton type Agammaglobulinemia
This is an X-linked immunodeficiency syndrome, due to failure of pre-B cells to differentiate into B cells. Clinically, the affected babies have multiple bacterial infections of the skin and respiratory system. There is some protection in first few months of life due to placental transfer of maternal IgG. Prognosis is fair with antibiotics and intravenous immunoglobulins, but children can die due to repeated lung infections leading to respiratory failure. Diagnosis is confirmed by the absence of B lymphocytes. The genetic defect is a mutation is in the tyrosine kinase gene (btk).
Deficiency of t cell development or function
DiGeorge Syndrome
In this syndrome children present with recurrent viral infections due to an abnormality in cellular immunity, characterized by reduced or absent T lymphocytes as a result of absence of the thymus gland. There are other congenital malformations noted in these children, like congenital heart disease and absent parathyroids. Individuals have tetany due to low serum calcium levels. Embryologically there are abnormalities of the third and fourth pharyngeal arches as a result of a deletion on chromosome 22 at 22q11.2. Routine chromosomal studies do not reveal this abnormality and fluorescent in situ hybridization technique is necessary for visualization of this defect.
COMBINED B AND T CELL DEFICIENCIES
Severe Combined Immunodeficiency (SCID)
SCID occurs due to a severe abnormality in cellular and humoral immunity, leading to increased susceptibility to bacterial and viral infections in infancy. SCID can be inherited as an X- linked form or autosomal recessive form due to adenosine deaminase or purine nucleoside phosphorylase deficiency.
The X-linked form occurs due to mutation in the lambda chain and the interleukin IL-2 receptor. In the autosomal recessive form, there is a deficiency of the enzyme adenosine deaminase or purine nucleoside phosphorylase. This deficiency occurs in more than 2/3 of the individuals. Other types of autosomal recessive forms of SCID occur due to mutations in the lymphocyte cytokine receptor or the T cell receptor. The class II genes of major histocompatibility complex are also associated with it. Children with the autosomal recessive form of SCID have cellular and humoral immunity with deficiency of granulocytes. Death occurs in the first year of life. Until recently the only available treatment was bone marrow transplant. However, today the treatment of choice is gene therapy and the first successful gene therapy recipient now being 9 yr of age.
Wiskott-Aldrich Syndrome
This is an X-linked disorder, and has several associated immunodeficiencies, in which T cells remain nonresponsive to antigens. Lymphocyte numbers are near normal, but the antibody is catabolized rapidly, showing abnormal substances. Affected males have eczema, diarrhea, repeated infections, thrombocytopenia and low Serum IgM levels bone marrow transplants are helpful in these cases, however death due to hemorrhage or B cell malignancies is common, especially in untreated cases.
Ataxia Telangectasia
This is a neurological disease associated with immunodeficiency. Children with ataxia telangectasia present with cerebellar ataxia (difficulty in control of movements and balance), dilated blood vessels of the face and conjunctiva, and pulmonary infections with a hypoplastic thymus. IgA levels are low.
Laboratory diagnosis is made by demonstration of low or absent serum IgA and IgG. Cytogenetic studies show characteristic abnormalities called chromosomal instability (Fig. 3). Individuals suffering from ataxia telangectasia have an increased risk of developing leukemia or lymphoid malignancies.
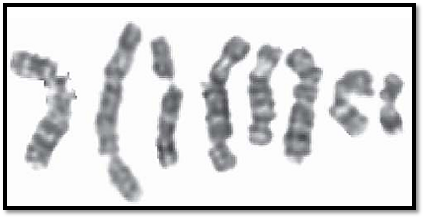
Fig. 3: Breaks observed in ataxia telangectasia
Blood Groups
Red cells have antigenic factors on their cell surface, and the significance of this led to safe blood transfusions, as well as prevention of Rhesus hemolytic disease of the newborn. So far about 400 blood group antigens have been described, and of these the best known are the ABO and Rhesus blood group systems.
ABO BLOOD GROUPS
Landsteiner in 1900 discovered ABO blood groups, when it was observed that sometimes transfusion of red blood cells from some persons to other leads to rapid hemolysis suggesting their blood was incompatible.
Four major blood groups have been identified A, B, AB and O. Individuals with A blood group have antigen A on the surface, B with have antigen B, AB have A and B antigens, while individuals with blood group O have neither. This means individuals with A blood group have anti B antibodies, B will have anti A and with O have both anti A and anti B antibodies in their blood.
ABO blood groups are an example of co-dominance. This means that alleles at the ABO blood group locus for antigens A and B are inherited in a co-dominant manner, but are dominant to the gene for the O antigen. Blood groups thus have genotypes which are homo and heterozygous for A and B which means we can have AA, AO, BB, BO.
Individuals with blood group AB will not produce A or B antibodies and therefore can receive blood transfusion from individuals of all ABO blood groups. They are therefore called universal recipients. Similarly individuals with O blood group do not express A or B antigens on red cells and are referred to as universal donors.
Molecular Aspects of ABO Blood Groups
Basic blood groups A, B and AB have enzymes with glycosyltransferase activity, which converts a basic blood group known an antigen H, into A or B antigen. A and B blood groups have a difference of seven single base substitutions which result in different A and B transferase activities. The A allele is associated with the addition of an N acetyl galactoseaminyl group and the B allele with a D galactosyl group. The O allele results from single base pair deletion resulting in an inactive protein, which cannot alter the H antigen.
Most people secrete the ABO blood group into body fluids like saliva ,sweat, plasma and semen. This is due to a secretor locus on the short arm of chromosome19.There are two alleles, Se, and se, hence three genotypes are possible. SeSe, Sese, and sese. The recessive homozygotes are unable to secrete their blood group substances into body fluids. This is thus determined as an autosomal dominant trait.
Rhesus blood group
The Rhesus (Rh) system is of importance in clinical practice because of its role in hemolytic disease of the newborn, as well as incompatibility arising in blood transfusion. The system was discovered by conducting experiments on Rhesus monkeys, thus the name Rh. Phenotypically there are two main rhesus blood groups, Rhesus positive and Rhesus negative. Rhesus positive individuals possess Rhesus antigen on their red cells and other tissues. Rhesus negative persons do not possess the same. The location of the rhesus group gene complex is on chromosome 1. Rh alleles are closely linked, and each allele has a specific polypeptide chain with multiple antigenic sites. Rh alleles have approximately eight antigenic combinations.
In clinical practice, the main significance of the Rh system is that Rh negative persons form anti Rh antibodies against Rh positive red blood cells. One has to remember that Rh negative females must be given Rh negative blood. If an Rh negative woman is pregnant and the father of the child is Rh positive, she can develop antibodies which in turn can produce hemolysis of the fetal red blood cells. This risk can be minimized by giving Rh immunogolulin injections during pregnancy, delivery, and after fetal tissue sampling, especially when the placenta is intervened, as in chorionic villus sampling and fetal blood sampling or in termination of the pregnancy.
Hemolytic Disease of the Newborn (HDN)
This disorder, once considered to be the most common genetic disease, has now become rare, as Rh immunoglobulin injections are readily available. During pregnancy, small volumes of fetal blood crosses the placental barrier to reach maternal blood stream stimulating the maternal cells to form antibodies. In HDN, due to these antibodies produced by maternal blood, fetal red blood cells are damaged, resulting in serious consequences. HDN can occur due to either Rh incompatibility where mother is Rh negative and the fetus Rh positive, or ABO incompatibility where mother is O and the fetus is type A or B. This is much milder and no treatment is required.
Transplantation Genetics
Organ transplantation has an become an integral part of clinical medicine. Corneal grafts and bone grafts are easily accepted by the body, but for other organ transplants, it is essential to have antigenic similarity between the donor and the recipient, otherwise there is rejection of the graft. Immune rejection remains the major barrier to successful tissue and organ transplantation. The basis for this is that the major histocompatibility complex (MHC) molecules, which all T cells must recognize in order to respond to foreign or abnormal peptide antigens are highly polymorphic in the human population. These are described below.
The major histocompatibility complex (MHC)
The major histocompatibility complex is a highly polymorphic gene cluster on the short arm of chromosome 6, which is responsible for regulating immune response. The genes for this cluster are 70 closely linked loci, and are called the HLA system. These code for the human leukocyte or HLA antigens.
These are divided into class I (A B C E F and G), class II (DR, DQ, and DP) and class III genes.
Human leukocyte antigen (HLA) system
The HLA system consists of highly polymorphic sites, hence phenotypic variation is high. The HLA phenotype of two unrelated individuals is highly unlikely to be exact. Various serological methods are used to define HLA patterns of an individual. Currently to this group of tests, DNA diagnostic tests have been added. As the HLA loci are closely linked they are inherited en block. HLA alleles of an individual are called haplotypes, and will be present on both the number 6 chromosomes. Due to segregation at meiosis each will have a 25% chance of sharing the same gene with a sib thus antigenically have more similarity than the parents, and therefore preferred for organ transplantations.
Hla and disease
Due to the extensive polymorphism of MHC molecules, they can serve as markers for disease susceptibility, if a disease gene were linked to the MHC. The different antigen presenting abilities of different individual MHC molecules might protect some individuals from disease while making others highly susceptible.
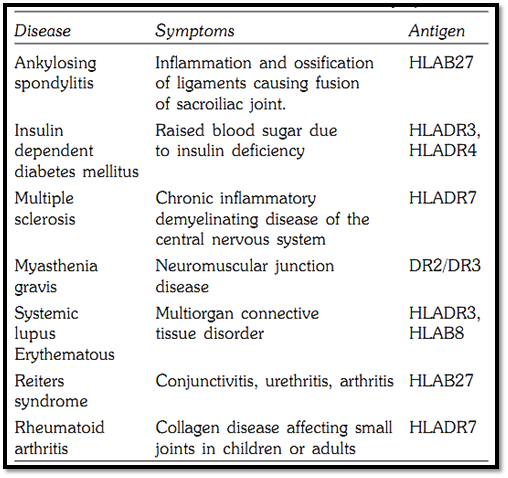
Certain HLA antigens show striking associations with certain diseases Table 2. The best known is the association of ankylosing spondylitis with HLAB27. While only 7% of the population has B27, the frequency in patients with ankylosing spondylitis is 95%. Other significant associations include HLADR3 and HLADR4 in insulin dependant diabetes mellitus, HLADR2 in multiple sclerosis, HLADR7 in psoriasis, HLAB27 in Reiter disease, HLADR7 in Rheumatoid arthritis and HLADR3 and HLAB8 in systemic lupus erythematosus.
Table 2: HLA associated diseases and symptoms
References
Purandarey, H. (2009). Essentials of Human Genetics. Second Edition. Jaypee Brothers Medical Publishers (P) Ltd.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
لأول مرة.. العتبة الحسينية تقيم صلاة العيد في مديرية مكافحة مخدرات بابل
|
|
|