


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 13-11-2015
Date: 11-12-2015
Date: 26-5-2016
|
Michael Faraday

Faraday and Davy
Among major scientists there has probably never been one so handsome, charming, and publicly popular as Humphry Davy. At the time he employed Faraday, he was at the peak of his celebrity. He made his headquarters at the Royal Institution, which had recently been founded by Count Rumford (Benjamin Thompson) for the “teaching, by regular courses and philosophical lectures and experiments, the applications of the new discoveries in science to the improvement of arts and manufactures, and in facilitating the means of procuring the comforts and conveniences of life.” During Davy's tenure as professor of chemistry at the institution, this social purpose became secondary to the professor's chemical research and famous scientific lectures.
Davy's lower-middle-class background was not far removed from Faraday's lower-class origins. His father was a wood carver, with a small farm in Penzance, Cornwall. He attended a good grammar school, but his formal education went no further. He found his interest in chemistry as an apprentice to a Penzance apothecary. Thomas Beddoes, a doctor in Clifton, Bristol, gave Davy his first scientific opportunity. Beddoes appointed Davy as the superintendent of experiments at his Medical Pneumatic Institution in Clifton. Davy's experiments with gases, particularly his descriptions of the effects of breathing “laughing gas” (nitrous oxide) “ a sensation analogous to gentle pressure on all the muscles, attended by a highly pleasurable thrilling, particularly in the chest and extremities” quickly became famous.
Davy's daring experiments and speculations caught Rumford's attention, and in 1799 Rumford appointed Davy to his first position at the Royal Institution, which became a platform for his aspirations in both science and society. In 1812, he married a wealthy and attractive widow, Jane Apreece. “Her passion for rank was as intense as Davy's,” writes one of Davy's biographers, J. G. Crowther. “The two social hunters allied in the attack on the aristocratic stockade.” For Davy, “the pursuit of science was rapidly subordinated to the pursuit of snobbery.”
Soon after Faraday started his scientific apprenticeship with Davy in 1813, he had another fortunate opportunity. The Davys, now Sir Humphry and Lady Davy, embarked on a tour of Europe, accompanied by Faraday as Davy's “assistant in experiments and writing.” The European tour was another essential part of Faraday's education, scientific and otherwise. Davy's fame opened doors everywhere in France and Italy, and Faraday met many of the leading scientists of the time. Davy himself was part of the education. He and his eager assistant freely discoursed on topics covering the scientific map and beyond. Lady Davy was a different matter. She talked too much and insisted on treating Faraday as a servant. “She is haughty and proud to an excessive degree and delights in making inferiors feel her power,” Faraday wrote to his friend Benjamin Abbott.
Faraday's European experience was as important as any other in his life. Williams tells us that “the young man who landed on English soil in the spring of 1815 was quite different from the youth who had left it in 1813. He had seen a good part of the world, realized its complexity and diversity, and gained a good deal of insight into the ways of men. He had met some of the foremost scientists of the day and had both impressed and been impressed by them.”
Discoverer
Most of Faraday's many biographers have portrayed him as a peerless discoverer of experimental facts. This image is certainly accurate as far as it goes, but it neglects another, equally important, side of his genius: his remarkable achievements as a theorist, or “philosopher,” as he preferred to be called. We first see him playing the familiar role of the experimentalist.
Faraday's early work at the Royal Institution, while Davy was still active in the institution's affairs, was mainly as a chemist. His first scientific paper, “Analysis of Native Caustic Lime of Tuscany,” was published in 1816 when he was twenty-five. By 1820, he had become a journeyman chemist, in demand for his services as an analytical chemist. During the 1820s, he helped keep the institution afloat financially by doing hundreds of chemical analyses. Also in the 1820s, Faraday turned to the topics of his major research, electricity and electromagnetism. Here we find him becoming the outstanding experimentalist of the nineteenth century.
The event that inspired Faraday's interest in electricity and magnetism was a discovery in 1820 by the Danish scientist, Hans Christian Oersted. The experiment was first performed as a demonstration before an audience of scientists. As Oersted described it later (using the third person to refer to himself),
The plan of the first experiment was, to make the [electrical] current of a little galvanic trough apparatus [a battery], commonly used in his lectures, pass through a very thin platina wire, which was placed over a compass covered with glass. The preparations for the experiment were made, but some accident having hindered him from trying it before the lecture, he intended to defer it to another opportunity; yet during the lecture, the probability of its success appeared stronger, so that he made the first experiment in the presence of the audience. The magnetical needle [the compass], though included in a box, was disturbed; but as the effect was very feeble . . . the experiment made no strong impression on the audience.
Faraday and others were more impressed. Oersted's experiment was a major event in the inauguration of the science of electromagnetism, which would ultimately lead to some of the technologies that are most familiar in our own lives. Faraday paid particular attention to Oersted's demonstration, later in 1820, that a current-carrying wire is surrounded by a circular magnetic effect, which forces the compass needle to point in a direction perpendicular to the wire.
Faraday guessed that a current-carrying wire could keep a magnet revolving in continuous circular motion around the wire's axis, and he designed the experiment illustrated in figure 1.1 to demonstrate this “electromagnetic rotation.” The left side of the figure shows a mercury-filled cup with a stationary electric current carrying wire dipping into it. A small, powerful magnet was placed next to the wire and tethered to the bottom of the cup by a thread. When an electric current was passed through the wire (and the mercury in the cup), the upper pole of the magnet rotated around the wire. The right side of the figure shows a similar experiment in which the magnet was fixed and the current-carrying wire rotated.
These experiments were reported in October 1821 and the paper “thrust Faraday into the first rank of European scientists,” writes Williams. “In every laboratory throughout Europe copies of Faraday's rotation apparatus were made an the strange nature of motive force contemplated.” Faraday's device had obvious practical possibilities: he had invented the electric motor. He did not pursue these applications, or any others made possible by his inventions. But others did.
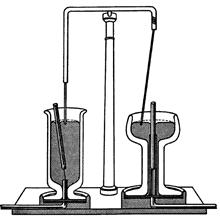
Figure 1.1. Faraday’s experiments demonstrating electromagnetic rotation.
By the 1830s, the performance of practical “electromagnetic engines” was being studied by James Joule, among others.
The 1821 paper and its immediate reception were an occasion for celebration and as it turned out, for a plagiarism charge against Faraday. The controversy concerned earlier unsuccessful and unpublished attempts by William Wollaston and Davy, to make a current-carrying wire rotate around its own axis when influenced by a magnet. This was not the same as Faraday's experiment, but it was similar enough that Faraday, who was familiar with the Wollaston-Davy effort, should have acknowledged it. In his haste to publish, he did not, and suspicions were aroused. Wollaston eventually allowed the storm to blow over, but Davy was not so magnanimous. When Faraday was proposed for election as a fellow of the Royal Society three years later, he had Wollaston's support but not that of Davy. Faraday was elected with one vote in opposition, no doubt Davy's vote. The master had broken with the pupil, evidently motivated to some degree by jealously and vanity.
Oersted's experiment was not the first to display an electromagnetic phenomenon. Earlier, Francois Arago and Andre Marie Ampere had demonstrated that a helical coil of wire carrying an electric current becomes a magnet, an “electromagnet.” In a series of experiments reported in 1831, Faraday investigated this connection between electricity and magnetism mediated by a coil of wire. He discovered the effect he eventually called “electromagnetic (or magneto-electric) induction.” The induction took place between two coils of wire wound around an iron ring, one coil carrying an electric current and serving as an electromagnet and the other connected to a copper wire that passed over a compass needle. Here is Faraday's typically meticulous description of the experiment, as recorded in his laboratory notebook:
I have had an iron ring made (soft iron), iron round and 7⁄8ths of an inch thick, and ring six inches in external diameter. Wound many coils round, one half of the coils being separated by twine and calico; there were three lengths of wire, each about twenty-four feet long, and they could be connected as one length, or used as separate lengths. By trials with a trough [a voltaic battery,] each was insulated from the other. Will call this side of the ring A [see fig. 1.2]. On the other side, but separated by an interval, was wound wire in two pieces, together amounting to about sixty feet in length, the direction being as with the former coils. This side call B.
Charged a battery of ten pairs of plates four inches square. Made the coil on
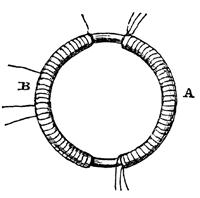
Figure 1.2. Faraday’s first electromagnetic induction experiment.
B side one coil, and connected its extremities by a copper wire passing to a distance, and just over a magnetic needle (three feet from wire ring), then connected the ends of one of the pieces on A side with battery: immediately a sensible effect on needle. It oscillated and settled at last in original position. On breaking connection of A side with battery, again a disturbance of the needle.
When it was connected to the battery, coil A became an electromagnet whose magnetic effect induced an electric current in coil B, as indicated by the magnetic needle (a compass). Faraday's key discovery, which had been missed for years by Faraday himself and many others, was that the induced electric current was transient: it lasted only for a short time after coil A was connected. In other words, the induction was in effect only while the magnetic effect was changing. Another transient current was induced in coil B when coil A was disconnected from the battery.
Oersted's experiment displayed a magnetic effect caused by an electric effect. Faraday's first induction experiment demonstrated the inverse, an electric effect caused by a magnetic effect, with the latter originating in an electromagnet. In another induction experiment, Faraday got a similar result by replacing the electromagnet with a permanent magnet. He wound a helical coil of wire around a hollow pasteboard cylinder, connected the coil to a galvanometer (for measuring electrical currents), and rapidly thrust a cylindrical permanent magnet into the cylinder. While the magnet was in motion—but only while it was in motion the galvanometer indicated that an electric current was induced in the coil.
Like the electromagnetic rotation experiments of 1821, Faraday's 1831 electromagnetic induction experiments had some obvious practical implications, and as usual, Faraday did not exploit them. The induction experiments showed that all one needed to produce electricity was a magnet and a coil of wire. The machines we now call dynamos or electric generators are based on this principle.
The 1830s were prolific years for Faraday. Soon after completing the electromagnetic induction experiments, he embarked on another profoundly important series of experiments, this one focusing on electrochemical decomposition, an interest he had inherited from Davy. In the prototype experiment of this kind, an electric current from a voltaic battery is passed through water, and the gases hydrogen and oxygen are evolved at the two wires making the electrical connection to the water. The chemical reaction promoted by the current is the decomposition of water (H2O) into hydrogen (H2) and oxygen (O2),
2 H2O → 2 H2 + O2.
This effect was first observed in 1800, and by the time Faraday turned to electrochemistry in the 1830s, many more electrochemical decompositions had been observed.
Faraday first concluded that in all cases the amount of chemical decomposition produced was proportional to the amount of electricity producing the effect. He also observed that the masses of elements liberated by a definite quantity of electricity were proportional to their chemical equivalent weights. (The equivalent weight of an element is about equal to the mass that combines with one gram of hydrogen. For example, the equivalent weight of oxygen in H2O is eight grams.)
From the second observation, Faraday concluded that “the equivalent weights of bodies are simply those quantities of them which contain the same quantity of electricity, or have naturally equal electrical powers; it being the electricity which determines the equivalent [weight], because it determines the combining force.” These statements, written in 1834, were astonishingly prophetic. They anticipated by more than fifty years the theories developed by physical chemists at the end of the nineteenth century based on the notion that in solution many chemical substances can dissociate into electrically charged components, each with its own equivalent weight.
With even greater prescience, Faraday continued: “Or, if we adopt the atomic theory or phraseology, then the atoms of bodies which are equivalents to each other in their ordinary chemical action, have equal quantities of electricity naturally associated with them.” Here he formulated the concept of charged particles in aqueous solutions, the “ions” of modern solution theory. But, like many of his contemporaries, he had reservations about atomism: “I must confess I am jealous of the term atom; for though it is very easy to talk of atoms, it is very difficult to form a clear idea of their nature, especially when compound bodies are under consideration.” With the gift of hindsight, we wonder why Faraday was not bold enough to believe in charged atoms, and even in an atom of electricity (the electron). This was a step he could not take because it violated his dictum that a postulate is not a truth unless it has the support of (many) experimental facts. Nothing was more important to Faraday than that.
We can credit Faraday with the founding of the science of electrochemistry. He not only proposed the two fundamental laws of electrochemistry mentioned above, but also introduced the language of electrochemistry, such terms as “electrolyte,” “electrode,” “cathode,” “anode,” “cation,” “anion,” and “ion.” Faraday had help in the invention of these terms from William Whewell of Trinity College, Cambridge. As Crowther remarks, “The famous terminology of [electrochemistry] was chiefly due to Whewell's excellent etymological taste.”
From electrochemistry, Faraday turned in 1837 to electrostatics. He had the idea, which he could see confirmed in the evidence of electrochemistry, that when two electrically charged bodies influence each other the effect depends not only on the charge itself but also on the medium between the two bodies. He designed a device called a “capacitor” in modern terminology. It consisted of two concentric brass spheres separated electrically by shellac insulation. The device could be opened, and the space between the two spheres filled with different insulating materials, gases, liquids, or solids.
Faraday had two precisely identical capacitors of this design made. In a typical experiment, he filled one capacitor with air and the other with another substance, such as glass, sulfur, or turpentine. He then charged one capacitor electrically and connected it with the other, thus dividing the charge between the two capacitors. Finally, with an electrometer he measured the charges on the capacitors. He found that the capacitor filled with a solid material always held a higher charge than the one with air. This was clear evidence that the electrical interaction between two charged bodies involved not only the charge and the distance between the two bodies, but also the medium the “dielectric,” as Faraday called it occupying the space between the bodies. If the dielectric was solid, some of the charge was induced in the dielectric itself. For Faraday, these “electrostatic induction” experiments illustrated an intimate reciprocal connection between electric forces and the medium in which they were effective: the forces altered the medium, and the medium propagated the forces.
Faraday was always strong physically. In the mountains, he could easily walk thirty miles in a day, and he was incessantly busy at the Royal Institution. His tragic weakness was recurring “ill health connected with my head,” as he put it. Even as a young man, he had memory problems, and as he grew older he suffered from bouts of depression and headaches. “When dull and dispirited, as sometimes he was to an extreme degree,” his niece Constance Reid recalled, “my aunt used to carry him off to Brighton, or somewhere, for a few days, and they generally came back refreshed and invigorated.”
These symptoms increased in severity and frequency until, in 1840, at age forty nine, Faraday had a major nervous breakdown. Brighton vacations were no longer curative, and for four years he avoided most of his research activities. One can glimpse his desperate condition in a letter to his friend Christian Schonbein, in 1843: “I must begin to write you a letter, though feeling, as I do, in the midst of one of my low nervous attacks, with memory so treacherous, that I cannot remember the beginning of a sentence to the end hand disobedient to the will, that I cannot form the letters, bent with a certain crampness, so I hardly know whether I shall bring to a close with consistency.”
Nevertheless, he came back. In 1845, he was again in his laboratory and closing in on what was to be one of his crowning achievements. This work was initiated by a suggestion in a letter from William Thomson, then a Cambridge undergraduate. Thomson mentioned the effects of electricity on dielectrics, already familiar to Faraday, and then offered the speculation that the electrical constraint of a transparent dielectric might have an effect on polarized light passing through the dielectric.
The phenomenon of light polarization had been known for many years. It was observed particularly in reflected light, and understood as a process that confined the vibrations constituting light waves to a certain plane. About a decade before Thomson's letter, Faraday had tried to detect a change in the plane of polarization of a light beam passing through a dielectric strained by electric charge. He got only negative results then. Faraday replied to Thomson, “Still I firmly believe that the dielectric is in a peculiar state whilst induction is taking place across it.” He was again inspired to search for the elusive effect, but modifications of the earlier search for electrical effects on polarized light were no more successful. It then occurred to him that a strong magnet might strain a solid dielectric sufficiently to affect the passage of a beam of polarized light.
In 1845, Faraday began a series of experiments based on this surmise. For the solids passing the polarized light, he tried flint glass, rock crystal, and calcareous spar; he varied the current supplied to his electromagnet, and the placements of the poles: still no success. He then tried a piece of lead glass he had prepared fifteen years earlier and at last the eureka moment arrived:
A piece of heavy glass . . . which was 2 inches by 1.8 inches, and 0.5 of an inch thick, being a silico borate of lead, and polished on the two shortest edges, was experimented with. It gave no effects when the same magnetic poles or th contrary poles were on opposite sides (as respects the course of the polarized ray) nor when the same poles were on the same side, either with the constant or intermitting current BUT, when contrary magnetic poles were on the same side, there was an effect produced on the polarized ray, and thus magnetic force and light were proved to have a relation to each other. This fact will most likely prove exceedingly fertile and of great value in the investigations of both conditions of natural forces.
Indeed. He had demonstrated a link between light and magnetism, the first step along the path that would lead to one of the greatest theoretical accomplishments of the nineteenth century, an electromagnetic theory of light, finally achieved by Maxwell building on Faraday's foundations.
Faraday never tired of telling his readers, correspondents, and audiences about the irreducible importance of tangible experimental facts. “I was never able to make a fact my own without seeing it,” he wrote to a friend toward the end of his career. In a letter to his colleague Auguste de la Rive, he recalled, “In early life I was a very lively imaginative person, who could believe in the ‘Arabian Nights’ as easily as in the ‘Encyclopaedia,’ but facts were important to me, and saved me. I could trust a fact.” The facts were the gifts of the experiments. “Without experiment I am nothing,” he said. And there was no end to the experiments: “But still try, for who knows what is possible?” To a lecture audience he said, “I am no poet, but if you think for yourselves, as I proceed, the facts will form a poem in your minds.” In the poetry, we find the other side of Faraday's genius.
Philosopher
The twentieth-century philosopher and historian Isaiah Berlin wrote a famous essay, “The Hedgehog and the Fox,” in which he classified thinkers as foxes or hedgehogs: foxes know many things, while hedgehogs know one big thing. Faraday was both. As an experimentalist, he learned all the things mentioned and a lot more (Bence Jones lists twenty-two topics pursued by Faraday in his electrical researches alone). But as a theorist, he learned and taught one great thing: that the forces of nature are all interconnected. “We cannot say that any one is the cause of the others, but only that they all are connected and due to a common cause,” he said in a lecture at the Royal Institution in 1834. In the 1845 paper reporting his discovery of the effect of magnetism on light, he wrote, “I have long held an opinion almost amounting to conviction . . . that the various forms under which the forces of matter are made manifest have one common origin; or, in other words, are so directly related and mutually dependent, that they are convertible, as it were, one into another, and possess equivalents of power in their action.” And in 1849 he said, “The exertions in physical science of late years have been directed to ascertain not merely the natural powers, but the manner in which they are linked together, the universality of each in its action, and their probable ”
These were not vague generalities. Faraday's experiments had given him a clear picture of natural forces. Magnetic forces could actually be mapped in the space surrounding a magnet by sprinkling iron filings on a piece of paper placed over the magnet. The filings aligned themselves along the magnetic “lines of force” (see fig. 1.3). Faraday assumed that the force between the magnetic pole was propagated along these lines. In 1849, Thomson introduced the now indispensable term “field of force,” or just “field,” for an entire network of Faraday's lines of force.
The iron filings showed that magnetic lines of force could be curved. In Oersted's experiment, they followed circles with the current-carrying wire at the center. The lines of force also determined the laws of electromagnetic induction. The rule was that if a wire cut through magnetic lines of force, an electric current was induced in the wire, and the magnitude of the current depended on the rate of cutting of the lines of force. Induction by this mode was particularly evident in Faraday's experiment with the helical coil of wire and the inserted magnet: as the magnet moved, the wire in the coil cut the lines of force carried by the magnet, and a current was induced.
Faraday generalized what he saw in the iron filings responding to a magnetic field to electric and gravitational fields. He had no device like the iron filings for developing an image of these lines of force, but he assumed that they were there, occupying the space even an otherwise empty space between interacting bodies.
All of this was a drastic departure from theoretical physics as Faraday found it in the early nineteenth century, which was based largely on a version of Newtonian physics. At the turn of the century, Newtonianism was unchallenged. The world was seen as a system of particles acting under forces that were manifested in the phenomena of electricity, magnetism, and gravitation. Each such force was transmitted instantaneously from one body to another without any mediating influence, and was determined mathematically by Newton's three laws. Theories of light were in a different category, but also reliant on particle models.
The first blow to the prevailing Newtonian view was struck by Thomas Young and Augustin Fresnel, who had by the 1830s demolished the particle theory of light and replaced it with a wave theory. The waves brought another problem. They were conceived as vibrations, but vibrations of what? To answer this question, theorists invented a strange kind of weightless matter called “ether” with some surprising properties: it could pass through ordinary matter completely without friction, and yet when called upon, it could support the extremely high frequencies of the vibrations of light waves.
The ether hypothesis was not the only theoretical device of the time to rely on weightless matter. A weightless fluid called “caloric” was popular in theories of heat, and electricity and magnetism were also treated as weightless fluids.
Faraday had little sympathy for any of these theoretical contrivances. He rejected
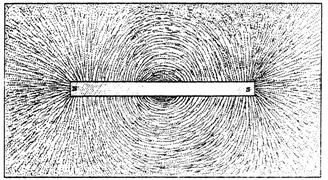
Figure 1.3. Magnetic lines of force traced by fine iron filings.
the ether concept and all weightless fluids, and refused to accept the Newtonian “action-at-distance” principle, which stated that the effect of a force, electric, magnetic, or gravitational, could reach from one body to another through empty space. In Faraday's worldview, space was occupied by fields comprising lines of force an electric field was generated by an electric charge, a magnetic field by the poles of a magnet, and a gravitational field by a massive object. Another body could respond to one of these fields, but not at a distance. The response was local, to the condition of the field where the second body was located. John Wheeler, a contemporary theoretical physicist, gives us a picture of a gravitational field that would meet with Faraday's approval:
The Sun, for instance, can be said to create a gravitational field, which spreads outward through space, its intensity diminishing as the inverse square of the distance from the Sun. Earth “feels” this gravitational field locally right where Earth is and reacts to it by accelerating toward the Sun. The Sun, according to this description, sends its attractive message to Earth via a field rather than reaching out to influence Earth at a distance through empty space. Earth doesn't have to “know” that there is a sun out there, 93 million miles distant. It only “knows” that there is a gravitational field at its own location. The field, though nearly as ethereal as the ether itself, can be said to have physical reality. It occupies space. It contains energy. Its presence eliminates a true vacuum. We must then be content to define the vacuum of everyday discourse as a region free of matter, but not free of field.
Faraday's theories were heretical and not popular with his contemporaries. “The reaction to the concept of the line of force was not merely one of indifference,” writes Williams, “it was downright hostile, especially when Faraday tried to extend it to gravitation. . . . The Athenaeum suggested that he go back to the Royal Institution and work up his sixth form mathematics before he ventured again into the deep seas of Laplacian physics.” In 1855, when he was sixty-four, Faraday said to his niece, Constance Reid, “How few understand the physical line of force! They will not see them, yet all the researches on the subject tend to confirm the views I put forth many years since. Thomson of Glasgow seems almost the only one who understands them. He is perhaps the nearest to understanding what I meant. I am content to wait, convinced as I am of the truth of my views.”
Faraday's theories were opposed because they were revolutionary, always sufficient reason to stir opposition, and also because Faraday did not speak the sophisticated mathematical language his fellow theorists expected to hear. Beyond rudimentary arithmetic, Faraday had no mathematics; his mathematical methods were about the same as those of Galileo. In Faraday's time, that may actually have been an advantage for creativity. The field concept was the product of “a highly original mind, a mind which never got stuck on formulas,” wrote a great twentieth-century field theorist, Albert Einstein. But for Faraday's audience theoretical physics had to be mathematical physics.
Faraday's great fortune was that he had two young followers who—apparently alone believed in the concepts of lines of force and field, and possessed all the equipment needed to build field theories in the requisite mathematical language. One of these mathematical physicists was William Thomson, as Faraday told his niece. To Faraday's great delight, Thomson formulated a mathematical theory of electric lines of force in 1845, when he was just twenty-one. The other mathematical physicist with his eyes on field theory was James Clerk Maxwell, who later, shortly before Faraday's death, created his great electromagnetic theory of light. Maxwell explained the genesis of his theory, and acknowledged his debt to Faraday and Thomson, in the preface to his Treatise on Electricity and Magnetism:
I was aware that there was supposed to be a difference between Faraday's way of conceiving phenomena and that of the mathematicians, so that neither he nor they were satisfied with each other's language. I had also the conviction that this discrepancy did not arise from either party being wrong. I was first convinced of this by Sir William Thomson, to whose advice and assistance, as well as to his published papers, I owe most of what I have learned on the subject.
As I proceeded with the study of Faraday, I perceived that his method of conceiving the phenomena was also a mathematical one, though not exhibited in the conventional form of mathematical symbols. I also found that these methods were capable of being expressed in the ordinary mathematical forms, and thus compared with those of the professed mathematicians.
Maxwell added that he deliberately read Faraday's Experimental Researches in Electricity before reading “any mathematics on the subject.”
Our account may give the impression that Faraday was first an experimentalist and then a theorist, in separate scientific lives, so to speak. But there was only one scientific life, a highly creative interplay between the experiments and the theoretical speculations. The experiments suggested the theories, and the theories guided the experiments. Neither endeavor would have succeeded without the other. This ability to work in the theoretical and experimental realms simultaneously and creatively is a rare gift. Only a few of the physicists in this book, perhaps only Newton and Fermi in addition to Faraday, had it. Einstein, Gibbs, Maxwell, Boltzmann, and Feynman were in the first rank of theorists, but not creative experimentalists.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|