


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 25-11-2021
Date: 10-11-2021
Date: 3-11-2021
|
α1-Antitrypsin in elastin degradation
Blood and other body fluids contain a protein, α1-antitrypsin (AAT), which inhibits a number of proteolytic enzymes (called peptidases, proteases, or proteinases) that hydrolyze and destroy proteins. [Note: The inhibitor was originally named AAT because it inhibits the activity of trypsin, a proteolytic enzyme synthesized as trypsinogen by the pancreas .] AAT has the important physiologic role of inhibiting neutrophil elastase, a powerful protease that is released into the extracellular space and degrades elastin of alveolar walls as well as other structural proteins in a variety of tissues (Fig. 1). Most of the AAT found in plasma is synthesized and secreted by the liver. Extrahepatic synthesis also occurs.
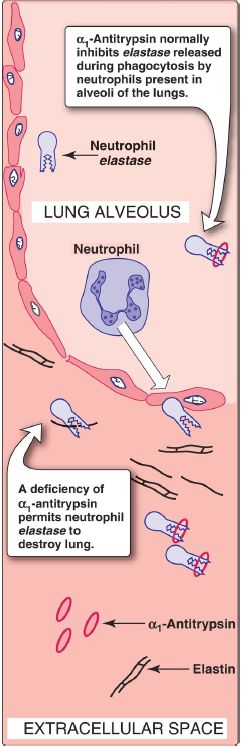
Figure 1: Destruction of alveolar tissue by elastase released from neutrophils activated as part of the immune response to airborne pathogens.
1. α1-Antitrypsin in the lungs: In the normal lung, the alveoli are chronically exposed to low levels of neutrophil elastase released from activated and degenerating neutrophils. The proteolytic activity of elastase can destroy the elastin in alveolar walls if unopposed by the action of AAT, the most important inhibitor of neutrophil elastase (see Fig. 1). Because lung tissue cannot regenerate, the destruction of the connective tissue of alveolar walls caused by an imbalance between the protease and its inhibitor results in pulmonary disease.
2. α1-Antitrypsin deficiency and emphysema: In the United States, ~2%–5% of patients with emphysema are predisposed to the disease by inherited defects in AAT. A number of different mutations in the gene for AAT are known to cause a deficiency of the protein, but one single purine base mutation (GAG to AAG, resulting in the substitution of lysine for glutamic acid at position 342 of the protein) is clinically the most widespread and severe. [Note: The mutated protein is termed the Z variant.] The mutation causes the normally monomeric AAT to misfold, polymerize, and aggregate within the RER of hepatocytes, resulting in decreased secretion of AAT by the liver. AAT deficiency is, therefore, a misfolded protein disease. Consequently, blood levels of AAT are reduced, decreasing the amount that gets to the lung. The polymer that accumulates in the liver may result in cirrhosis (scarring of the liver). In the United States, the AAT mutation is most common in Caucasians of Northern European ancestry. An individual must inherit two abnormal AAT alleles to be at risk for the development of emphysema. In a heterozygote, with one normal and one defective gene, the levels of AAT are sufficient to protect the alveoli from damage. [Note: Methionine 358 in AAT is required for the binding of the inhibitor to its target proteases.
Smoking causes the oxidation and subsequent inactivation of the methionine, thereby rendering the inhibitor powerless to neutralize elastase. Smokers with AAT deficiency, therefore, have a considerably elevated rate of lung destruction and a poorer survival rate than nonsmokers with the deficiency.] The deficiency of elastase inhibitor can be treated by weekly augmentation therapy, that is, intravenous administration of AAT. The AAT diffuses from the blood into the lung, where it reaches therapeutic levels in the fluid surrounding the lung epithelial cells.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
أصواتٌ قرآنية واعدة .. أكثر من 80 برعماً يشارك في المحفل القرآني الرمضاني بالصحن الحيدري الشريف
|
|
|