


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| Production of rRNA Requires Cleavage Events and Involves Small RNAs |
|
|
|
Read More
Date: 16-12-2015
Date: 20-12-2015
Date: 9-5-2021
|
Production of rRNA Requires Cleavage Events and Involves Small RNAs
KEY CONCEPTS
- RNA polymerase I terminates transcription at an 18-base terminator sequence.
- The large and small rRNAs are released by cleavage from a common precursor rRNA; the 5S rRNA is separately transcribed.
- The C/D group of snoRNAs is required for modifying the 2′ position of ribose with a methyl group.
- The h/ACA group of snoRNAs is required for converting uridine to pseudouridine.
- In each case the snoRNA base pairs with a sequence of rRNA that contains the target base to generate a typical structure that is the substrate for modification.
The major rRNAs are synthesized as part of a single primary transcript that is processed by cleavage and trimming events to generate the mature products. The precursor contains the sequences of the 18S, 5.8S, and 28S rRNAs. (The nomenclature of different ribosomal RNAs is based on early sedimentation studies conducted on sucrose gradients in the 1970s.) In multicellular eukaryotes, the precursor is named for its sedimentation rate as 45S RNA. In unicellular/oligocellular eukaryotes it is smaller (35S in yeast).
The mature rRNAs are released from the precursor by a combination of cleavage events and trimming reactions to remove external transcribed spacers (ETSs) and internal transcribed spacers (ITSs). FIGURE 1 shows the general pathway in yeast. The order of events can vary, but basically similar reactions are involved in all eukaryotes. Most of the 5′ ends are generated directly by a cleavage event. Most of the 3′ ends are generated by cleavage followed by a 3′–5′ trimming reaction. These processes are specified by many cis-acting RNA motifs in ETSs and ITSs and are acted upon by more than 150 processing factors.
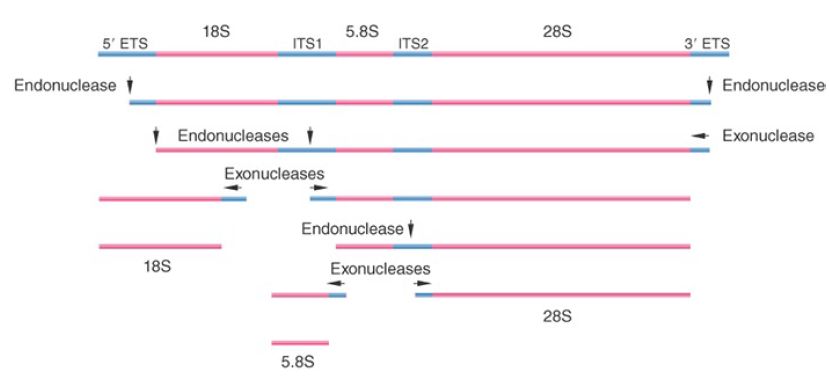
FIGURE 1.Mature eukaryotic rRNAs are generated by cleavage and trimming events from a primary transcript.
Many ribonucleases have been implicated in processing rRNA, including some specific components of the exosome, which is an assembly of several exonucleases that also participates in mRNA degradation . Mutations in individual enzymes usually do not prevent processing, which suggests that their activities are redundant and that different combinations of cleavages can be used to generate the mature molecules.
Multiple copies of the transcription unit for the rRNAs are always available. The copies are organized as tandem repeats . The genes encoding rRNAs are transcribed by RNA polymerase I in the nucleolus. In contrast, 5S RNA is transcribed from separate genes by RNA polymerase III. In general, the 5S genes are clustered, but are separated from the genes for the major rRNAs. In bacteria, the organization of the precursor differs. The sequence corresponding to 5.8S rRNA forms the 5′ end of the large (23S) rRNA; that is, no processing occurs between these sequences.
FIGURE 2 shows that the precursor also contains the 5S rRNA and one or two tRNAs. In Escherichia coli, the seven rrn operons are dispersed around the genome; four rrn loci contain one tRNA gene between the 16S and 23S rRNA sequences, and the other rrn loci contain two tRNA genes in this region. Additional tRNA genes may or may not be present between the 5S sequence and the 3′ end. Thus, the processing reactions required to release the products depend on the content of the particular rrn locus.
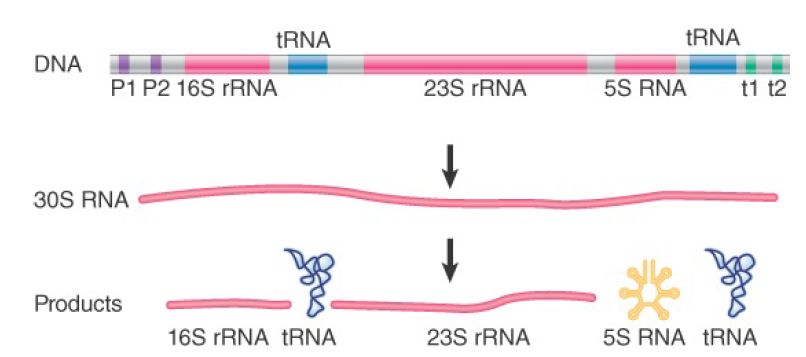
FIGURE 2.The rrn operons in E. coli contain genes for both rRNA and tRNA. The exact lengths of the transcripts depend on which promoters (P) and terminators (t) are used. Each RNA product must be released from the transcript by cuts on either side.
In prokaryotic and eukaryotic rRNA processing, both processing factors and ribosomal proteins (and possibly other proteins) bind to the precursor so that the substrate for processing is not the free RNA but rather a ribonucleoprotein complex. Like pre-mRNA processing, rRNA processing takes place cotranscriptionally. As a result, the processing factors are intertwined with ribosomal proteins in building the ribosomes, instead of first processing and then stepwise assembly on processed rRNAs.
Processing and modification of rRNA requires a class of small RNAs called small nucleolar RNAs (snoRNAs). The S. cerevisiae and vertebrate genomes have hundreds of snoRNAs. Some of these snoRNAs are encoded by individual genes; others are expressed from polycistrons; and many are derived from introns of their host genes. These snoRNAs themselves undergo complex processing and maturation steps. Some snoRNAs are required for cleavage of the precursor to rRNA; one example is U3 snoRNA, which is required for the first cleavage event. The U3-containing complex corresponds to the “terminal knobs” at the 5′ end of nascent rRNA transcripts, which are visible under an electron microscope. We do not know what role the snoRNA plays in cleavage. It could be required to pair with specific rRNA sequences to form a secondary structure that is recognized by an endonuclease.
Two groups of snoRNAs are required for the modifications that are made to bases in the rRNA. The members of each group are identified by very short conserved sequences and common features of secondary structure.
The C/D group of snoRNAs is required for adding a methyl group to the 2′ position of ribose. There are more than 100 2′-O-methyl groups at conserved locations in vertebrate rRNAs. This group takes its name from two short, conserved sequence motifs called boxes C and D. Each snoRNA contains a sequence near the D box that is complementary to a region of the 18S or 28S rRNA that is methylated. Loss of a particular snoRNA prevents methylation in the rRNA region to which it is complementary.
FIGURE 3 shows that the snoRNA base pairs with the rRNA to create the duplex region that is recognized as a substrate for methylation. Methylation occurs within the region of complementarity at a position that is fixed five bases on the 5′ side of the D box. It is likely that each methylation event is specified by a different snoRNA; about 40 snoRNAs have been implicated in this modification. Each C+D box snoRNA is associated with three proteins: Nop1 (fibrillarin in vertebrates), Nop56, and Nop58. The methylase(s) have not been fully characterized, although the major snoRNP protein Nop1/fibrillarin is structurally similar to
methyltransferases.
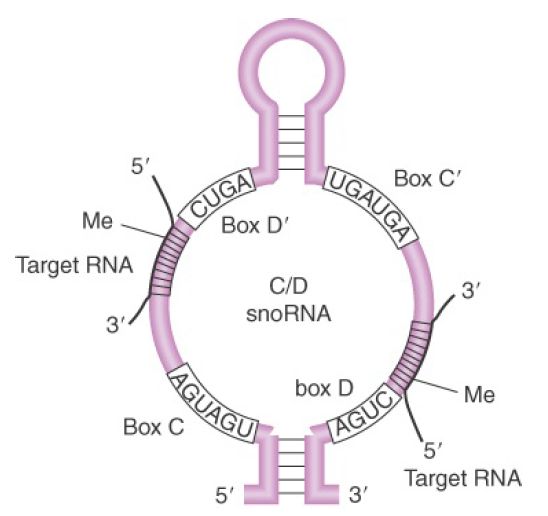
FIGURE 3. A snoRNA base pairs with a region of rRNA that is to be methylated.
Another group of snoRNAs is involved in base modification by converting uridine to pseudouridine. About 50 residues in yeast rRNAs and about 100 in vertebrate rRNAs are modified by pseudouridination. The pseudouridination reaction is shown in FIGURE 4, in which the N1 bond from uridylic acid to ribose is broken, the base is rotated, and C5 is rejoined to the sugar.
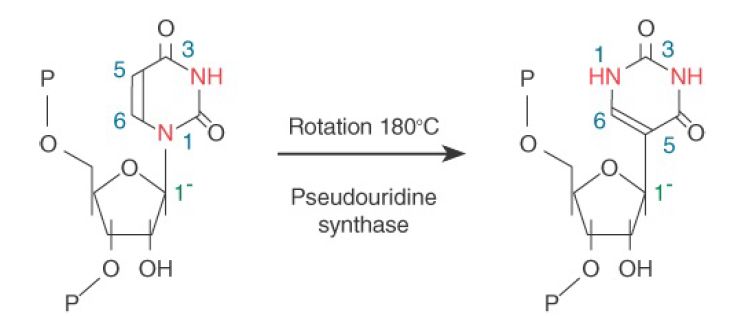
FIGURE 4. Uridine is converted to pseudouridine by replacing the N1-sugar bond with a C5-sugar bond and rotating the base relative to the sugar.
Pseudouridine formation in rRNA requires the H/ACA group of about 20 snoRNAs. They are named for the presence of an ACA triplet three nucleotides from the 3′ end and a partially conserved sequence (the H box) that lies between two stem-loop hairpin structures. Each of these snoRNAs has a sequence complementary to rRNA within the stem of each hairpin. FIGURE 5 shows the structure that would be produced by pairing with the rRNA. Each pairing region has two unpaired bases, one of which is a uridine that is converted to pseudouridine.
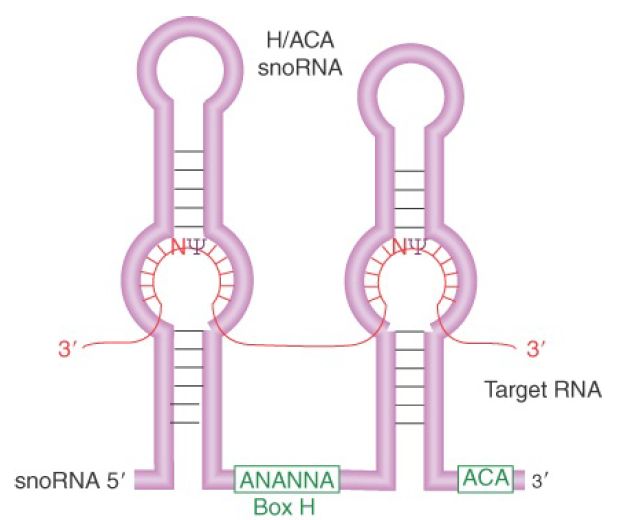
FIGURE 5. H/ACA snoRNAs have two short, conserved sequences and two hairpin structures, each of which has regions in the stem that are complementary to rRNA. Pseudouridine is formed by converting an unpaired uridine within the complementary region of the rRNA.
The H/ACA snoRNAs are associated with four specific nucleolar proteins: Cbf5 (dyskerin in vertebrates), Nhp2, Nop10, and Gar1. Importantly, Cbf5/dyskerin is structurally similar to known pseudouridine synthases, and thus it likely provides the enzymatic activity in the snoRNA-guided pseudouridination reaction. Many snoRNAs are also used to guide base modifications in tRNAs as well as in snRNAs involved in pre-mRNA splicing, which are critical for their functions in prospective reactions. However, a large number of snoRNAs do not have apparent targets. These snoRNAs are called orphan RNAs. The existence of these orphan RNAs indicates that many biological processes may use RNA-guided mechanisms to functionally modify other expressed RNAs in a more diverse fashion than we currently understand.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|