


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 24-12-2015
Date: 9-5-2016
Date: 30-4-2021
|
Sporulation Is Controlled by Sigma Factors
KEY CONCEPTS
- Sporulation divides a bacterium into a mother cell that is lysed and a spore that is released.
- Each compartment advances to the next stage of development by synthesizing a new sigma factor that displaces the previous sigma factor.
- Communication between the two compartments coordinates the timing of sigma factor substitutions.
A good example of the use of switching of holoenzymes to control changes in gene expression is provided by sporulation, an alternative lifestyle that occurs in many bacterial species. When logarithmic growth ceases because nutrients in the medium become depleted, the vegetative phase in growth of these bacteria ends. This triggers sporulation, a developmental stage in which the cell is resistant to many kinds of environmental and nutritional stresses (illustrated in FIGURE 1). During spore formation in B. subtilis, one of the daughter genomes that results from DNA replication is segregated at one end of the cell, attached to the cell pole. A septum forms, generating two independent compartments: the mother cell and the forespore. The growing septum traps part of one chromosome in the forespore, and then a translocase (SpoIIIE) pumps the rest of the chromosome into the forespore. Eventually the forespore, with its engulfed chromosome, is surrounded by a tough coat, and this spore is stable almost indefinitely.
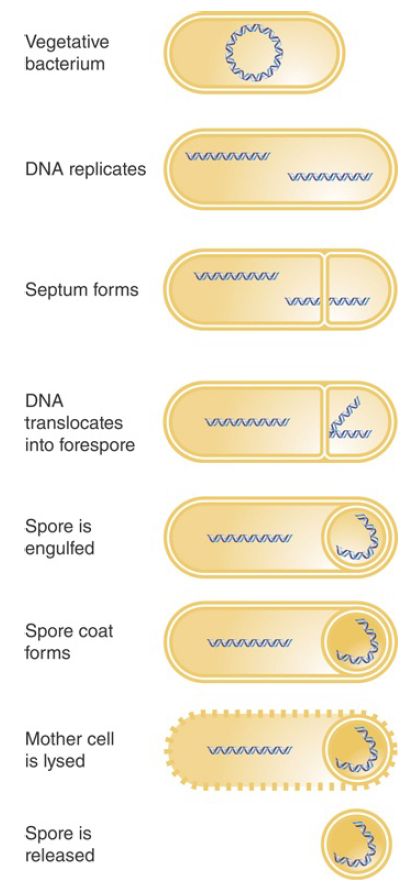
FIGURE 1. Sporulation involves the differentiation of a vegetative bacterium into a mother cell that is lysed and a spore that is released.
Sporulation takes approximately 8 hours. It can be viewed as a primitive sort of differentiation, in which a parent cell (the vegetative bacterium) gives rise to two different daughter cells with distinct fates: The mother cell is eventually lysed, and the spore that is released has an entirely different structure from the original bacterium.
Sporulation involves a drastic change in the biosynthetic activities of the bacterium, in which many genes are involved. Changes in gene expression resulting ultimately in the formation of the spore result primarily from changes in transcription initiation. Some of the genes that function in the vegetative phase are turned off during sporulation, but most continue to be expressed. Many genes specific for sporulation are expressed only during this period, though. At the end of sporulation, about 40% of the bacterial mRNA is sporulation specific. New forms of RNA polymerase become active in sporulating cells; they contain the same core enzyme as vegetative cells, but have different proteins in place of the vegetative sigma factor, σA . The changes in transcriptional specificity are summarized in FIGURE 2. The principle is that in each compartment the existing sigma factor is successively displaced by a new sigma factor that causes transcription of a different set of genes. Communication between the compartments occurs in order to coordinate the timing of the changes in the forespore and mother cell.
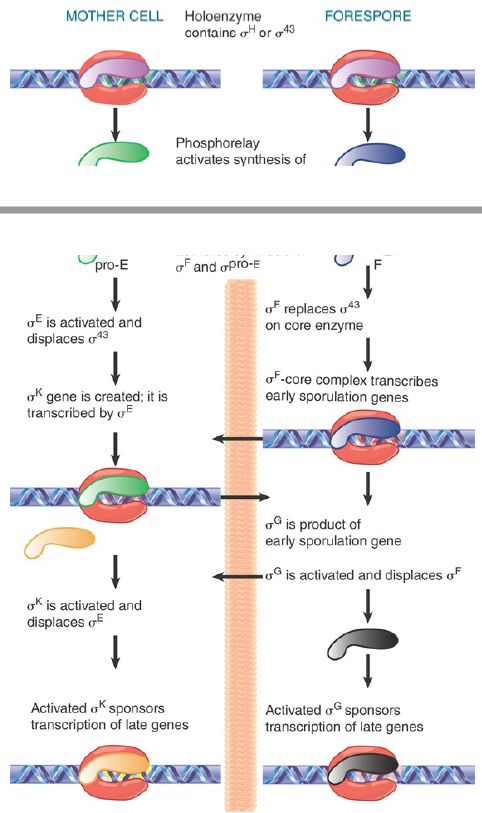
FIGURE 2. Sporulation involves successive changes in the sigma factors that control the initiation specificity of RNA polymerase. The cascades in the mother cell (left) and the forespore (right) are related by signals passed across the septum (indicated by horizontal arrows).
The sporulation cascade is initiated when environmental conditions trigger a phosphorelay, in which a phosphate group is passed along a series of proteins until it reaches a transcriptional regulator called SpoOA. Many gene products are involved in this process, whose complexity reflects the utilization of checkpoints—times when the bacterium confirms that it wishes to continue on the pathway to differentiation. This is not a regulatory course that should be undertaken unnecessarily, as the ultimate decision is irreversible.
Activation of SpoOA by phosphorylation marks the beginning of sporulation. In its phosphorylated form, SpoOA activates transcription of two operons, each of which is transcribed by a different form of the host RNA polymerase. Host enzyme utilizing the general sigma factor σA transcribes the gene coding for σF , and host enzyme under the direction of another sigma factor, σH , transcribes the gene encoding a precursor to the sigma factor σE .
The precursor sigma factor is referred to as pro-σE . Both σF and pro-σE are produced before septum formation, but become active later.
Transcription directed by σF is inhibited because an antisigma factor (SpoIIAB) binds to it, preventing it from forming a holoenzyme. In the forespore, however, an anti-antisigma factor (SpoIIAA) inhibits the inhibitor. Inactivation of the anti-antisigma is controlled by a series of phosphorylation/dephosphorylation events, in which dephosphorylation by a phosphatase called SpoIIE is the first step. SpoIIE is an integral membrane protein that accumulates at the cell pole, with the result that its phosphatase domain becomes more concentrated in the forespore. In summary, dephosphorylation activates SpoIIAA, which, in turn, displaces SpoIIAB from σF . Release of σF activates it.
Activation of σF marks the start of cell-specific gene expression. Under the direction of σF , RNA polymerase transcribes the first set of sporulation genes. Not all transcription in the forespore comes from σF -directed transcription. σA is not destroyed during sporulation, and, therefore, the vegetative holoenzyme, Eσ , remains in sporulating cells. (An “EσA” holoenzyme refers to the polymerase enzyme plus a given sigma factor.)
The cascade continues as products derived from promoters recognized by EσF are made in the forespore (see FIGURE 3). For example, EσF makes a transcript encoding σG , which, in turn, forms the holoenzyme that transcribes the late sporulation genes. EσF also recognizes a promoter controlling expression of a product responsible for communicating with the mother cell compartment, SpoIIR, which is secreted from the forespore into the membrane separating the two compartments. In the membrane, SpoIIR activates the membrane-bound protein SpoIIGA, which cleaves inactive precursor pro-σE into active σE in the mother cell. (σE produced in the forespore is degraded.)
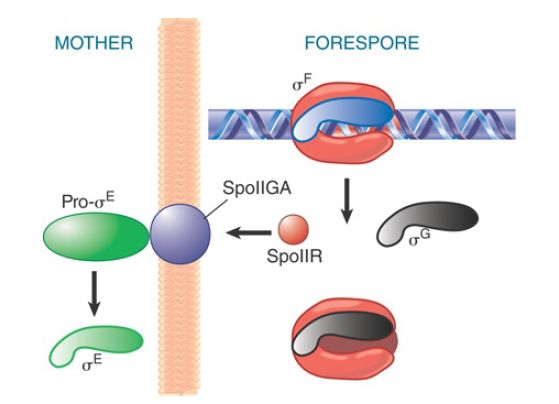
FIGURE 3. σF triggers synthesis of the next sigma factor in the forespore (σG ) and turns on SpoIIR, which causes SpoIIGA to cleave pro-σE .
The cascade continues when σE in the mother cell is replaced by σK . (The production of σK is quite complex, because its gene is created by a site-specific recombination event!) Like σE , σK is also synthesized as an inactive precursor, pro-σK . Thus, σK has to be activated by cleavage of its precursor form before it can replace σE and transcribe late genes in the mother cell. The timing of these events in the two compartments is coordinated by still other signals. In summary, the activity of σE in the mother cell is necessary for activation of σG in the forespore, and the activity of σG is required to generate a signal that is transmitted across the septum to activate σK .
Sporulation is thus controlled by a cascade in which sigma factors in each compartment are successively activated by sigmas F, E, G, and K, each directing the synthesis of a particular set of genes.
The cascade can be represented by a crisscross pattern of signals crossing the septum, connecting gene expression in one compartment with that in the other, as illustrated in FIGURE 4. As new sigma factors become active, old sigma factors are displaced, turning sets of different genes on and off in the two compartments.
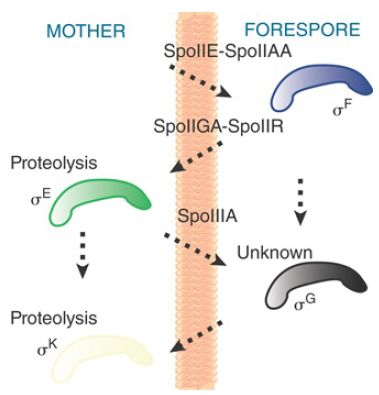
FIGURE 4. The crisscross regulation of sporulation coordinates timing of events in the mother cell and forespore.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|