


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 10-12-2015
Date: 18-5-2016
Date: 28-12-2015
|
Colony-Stimulating Factors
Colony-stimulating factors (CSFs) are glycoproteins that stimulate the proliferation, differentiation, and survival of hematopoietic (blood) cells and also activate mature myeloid cell functions. CSFs were first described in the 1960s as activities in various conditioned media that stimulated the growth of colonies of bone marrow cells in semisolid cultures. These colony-forming cells are the precursors of our red and white blood cells. Purification and characterization of these activities were difficult with the biochemical techniques available because they were present in conditioned media in very low concentrations and also because they were heterogeneous, largely due to variable glycosylation. Eventually, in the late 1970s and 1980s, four different murine CSFs were purified, followed by their human counterparts. These were called
1. Multi-CSF or interleukin-3 (IL-3(
2. Granulocyte-macrophage (GM)-CSF
3. Granulocyte (G)-CSF
4. Macrophage (M)-CSF, or CSF-1
Since then, the development of molecular biology has led to the cloning of CSF complementary DNAs and the production of purified recombinant proteins, facilitating in vitro and in vivo studies of their activities. All four CSFs have been tested in clinical trials, and both G-CSF and GM-CSF are used in clinical practice to elevate leukocyte levels in a variety of situations. In addition to these CSFs, many interleukins and growth factors have been described that stimulate or co-stimulate bone marrow cell colony growth. The most important of these are the early-acting stem cell factor (SCF( and FLT3/FLK-2 ligand (FL) (1), the erythroid lineage stimulator erythropoietin, and the megakaryocyte and platelet stimulator thrombopoietin (TPO). Recently, TPO has been found to have multilineage activities (2).
1. Biochemical Characteristics
The CSFs belong to a family of cytokines that have little sequence identity but were predicted to be similar in structure (3). The protein structures of all four have now been determined, although for IL-3 a mutant molecule with enhanced stability was used, rather than the native molecule (4). They are all four-helix bundles with up–up–down–down connectivity. IL-3, G-CSF, and GM-CSF are monomeric secreted proteins, but the soluble form of M-CSF is a disulfide-bonded homodimer produced by proteolytic cleavage of cell-membrane molecules. Other characteristics are given in Table 1.
Table 1. Biochemical Characteristics of the CSFs
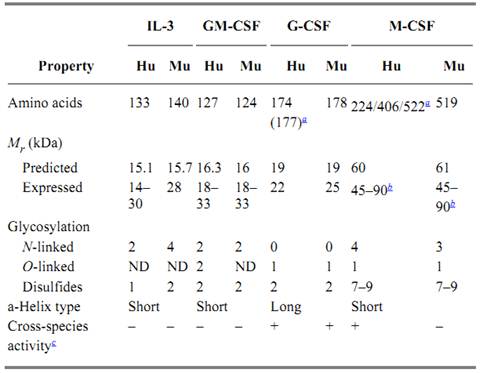
a Different forms resulting from alternative splicing.
b Disulfide-bonded dimer of proteolytically cleaved transmembrane protein.
c Between human and murine.
2. CSF Genes and Their Expression
The cloning of genomic DNA encoding the CSFs and analysis of the regions 5′ of the genes has resulted in a better understanding of the control of CSF expression (see Table 2). A single messenger RNA species is transcribed for IL-3 and GM-CSF, whereas a minor alternatively spliced mRNA is produced for human G-CSF that results in a three-amino-acid-residue insertion in the protein after residue Leu35 and a 20-fold reduction in activity. Transcription of M-CSF mRNA is more complex, with four species resulting from alternative splicing of the 10 exons. The largest two differ only in the 3′ untranslated region and encode identical 522-residue transmembrane proteins. The smaller two result from splicing out of part of exon 6, which results in loss of the proteolytic cleavage site from the protein produced from the 1.6-kb transcript and expression of a stable transmembrane protein (5). The DNA regions upstream of the coding regions contain promoter and enhancer elements that have been partially characterized and shown to respond to CSF inducers such as tumor necrosis factor (TNF), IL-1, lipopolysaccharide (LPS), and antigens. The control of CSF gene expression is not understood completely, but it involves control of mRNA stability by elements in the 3′ untranslated region of the mRNAs, as well as control of transcription.
Table 2. Characteristics of the Genes Encoding the CSFs
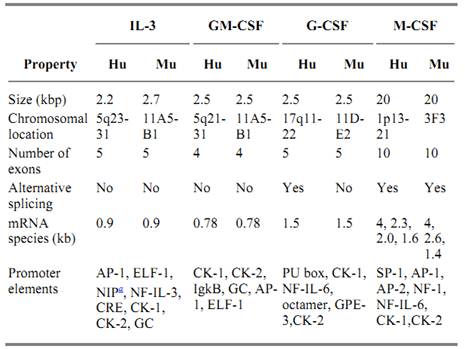
a Repressor element. Data from Refs. 18 and 19.
3. CSF Production
Many cell types have been shown to produce CSFs in vitro in response to various stimuli, such as bacterial products (LPS) and inflammatory cytokines (TNF, IL-1), but the role of CSFs in maintaining white blood cell levels in normal healthy individuals is not entirely clear. Whether unstimulated cells produce CSFs is still debated (6). GM-CSF, G-CSF, and M-CSF are produced by macrophages, endothelial cells, fibroblasts, and stromal cells. In addition, T lymphocytes produce GM-CSF, M-CSF (in humans), and IL-3. Mast cells have been shown to produce IL-3 and GM-CSF. Other cell types have been reported to produce GM-CSF and IL-3, but these studies have yet to be confirmed.
4. CSF Receptors
The CSF receptors are transmembrane proteins belonging to the cytokine receptor class I family, except for the M-CSF receptor, which is a tyrosine kinase receptor. The class I receptors contain a structurally conserved module called the cytokine-binding domain (CBD), the hemopoietin domain, or the cytokine receptor homology domain. This module comprises two fibronectin type III domains with four conserved cysteine residues in the N-terminal domain and a conserved Trp Ser X TrpSer sequence in the C-terminal domain. Ligand binding causes dimerization or oligomerization of the receptors, which initiates signal transduction. The IL-3 and GM-CSF receptors form heterodimers of a ligand-specific a-chain and a common b-chain that is also shared with the IL-5 receptor. In the mouse, there is an additional b-chain that is unique to the IL-3 receptor. The G-CSF and M-CSF receptors form homodimers. Receptor dimerization activates tyrosine kinases (intrinsic in the M-CSF receptor, cytoplasmic JAK kinases for the other receptors) that phosphorylate the receptors, providing binding sites for other signaling molecules that are in turn activated by phosphorylation of tyrosine residues and, in some cases, serine or threonine. Ultimately, these signaling cascades lead to alterations in gene transcription, but the components of the biochemical pathways are still being defined. The CSF receptors are expressed at low levels on hematopoietic cells, usually a few hundred receptors per cell. IL-3 receptors are expressed on neutrophil, eosinophil, and macrophage lineages, on mast cells, and on undifferentiated blast cells. GM-CSF receptors are expressed on neutrophil, eosinophil, and macrophage lineages. G- and M-CSF receptor expression is more restricted; G-CSF receptors are present on neutrophils and at a low level on macrophages, and M-CSF receptors are found on macrophages, osteoclasts, and placental trophoblasts. Recent studies have detected expression of receptors on purified stem-cell populations. Human CD34+ cells expressed SCF, FL, G-CSF, and, at a low level, GM-CSF receptors (10). Murine stem cell populations expressed IL-1a, IL-3, IL-6, and G-CSF receptors (11).
5. Biological Activity
5.1 n Vitro
All four of the CSFs stimulate the proliferation and differentiation of bone-marrow progenitors to form colonies in semisolid agar. The type of mature cells in the colonies varies with the stimulator ( see Fig. 1). IL-3 was otherwise called multi-CSF because it stimulates the largest number of different cell lineages. Colonies of granulocytes, macrophages, eosinophils, megakaryocytes, undifferentiated blast cells, and mixed cell lineages are obtained. In liquid cultures, proliferation of mast cells is strongly stimulated by IL-3, and some types of B lymphocytes are reported to respond. GM-CSF stimulates growth of granulocytic, macrophage, and eosinophil colonies. Production of dendritic cells, which are important antigen-presenting cells in the immune response, is stimulated by GM-CSF. G-CSF and M-CSF are more lineage restricted, producing mainly granulocytic and macrophage colonies, respectively. The CSFs can give enhanced or synergistic responses when used in combination with each other and with other interleukins and growth factors. Some effects of CSFs on nonhematopoietic cells have been reported. G- and GM-CSF can stimulate proliferation of endothelial cells. Placenta-derived cells and oligodendrocytes proliferate in response to GM-CSF. M-CSF stimulates proliferation of trophoblast cells and may be important in pregnancy.
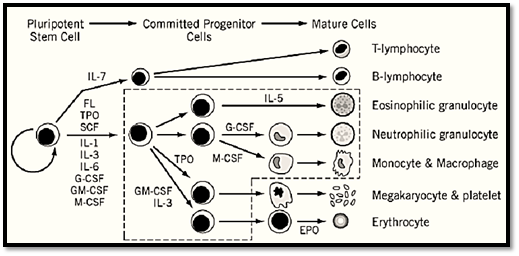
Figure 1. Schematic representation of hematopoiesis and the CSFs and other cytokines required for mature cell production. IL, interleukin; FL, FLT3/FLK-2 ligand; SCF, stem cell factor; TPO, thrombopoietin. GM-CSF and IL-3 stimulate cells inside the dotted line box.
5.2. In Vivo
The effects of CSFs in vivo have been determined both by injection in mice and humans and by analysis of CSF-deficient mice created by gene targeting and, in the case of M-CSF, a naturally occurring mutation. Injection of IL-3 or GM-CSF elevates circulating levels of neutrophils, monocytes, and eosinophils. G-CSF causes a greater elevation of neutrophils than does IL-3 or GM-CSF, but only a weak increase in monocytes and no effect on other lineages. M-CSF causes a selective elevation of monocytes. The magnitude of these increases depends on the dose of CSF administered. It is interesting that in response to infection, circulating IL-3 or GM-CSF are not usually detected, whereas G-CSF and, to a lesser extent, M-CSF are frequently elevated in serum. These observations suggest that IL-3 and GM-CSF may be important in local inflammatory responses in the tissues rather than in a systemic response to infection.
The importance of the CSFs in maintaining basal hematopoiesis has been assessed by analysis of CSF-deficient mice. For IL-3, GM-CSF, and G-CSF, gene “knockout” mice were created by introduction of a defective version of the gene of interest into embryonic stem cells by homologous recombination. The embryonic stem cells were injected into blastocysts to create chimeric mice that were then bred to create heterozygous and homozygous deficient mice. For M-CSF, mice with a naturally occurring point mutation (op/op) that resulted in a frameshift in the sequence and a loss of M-CSF production were discovered. The IL-3- and GM-CSF-deficient mice were able to produce normal levels of circulating blood cells and bone marrow progenitors. The GM-CSF-deficient mice developed pulmonary alveolar proteinosis and increased pulmonary infections, presumably as a consequence of defective pulmonary macrophage function. Recent studies in IL-3-deficient mice have shown that IL-3 is important for elevation of basophils and mast cells in response to a parasitic infection (12) and for some delayed-type hypersensitivity (T-lymphocyte-dependent) responses (13). G-CSF defective mice were neutropenic, with neutrophil numbers reduced to 20–35% of wild-type control mice. Bone marrow cellularity was normal, but some granulocytic and macrophage progenitors were reduced in number. These mice were more susceptible to a bacterial infection (Listeria monocytogenes) than wild-type mice. Young M-CSF-deficient mice have osteopetrosis, a severe macrophage and osteoclast deficiency resulting in failure of teeth eruption and overgrowth of bone that occludes the marrow cavity and reduces marrow cellularity. Unlike the other CSF-deficient mice, the op/op mice overcome their defects with age, which suggests that other cytokines may be able to compensate for the lack of M-CSF. Thus, the CSF-defective mice have shown that each CSF has a unique function in vivo. GM-CSF and IL-3 are not required for steady-state hematopoiesis. G- and M-CSF are required for normal neutrophil and macrophage production, respectively. All four CSFs contribute in different ways to control of infection. They may have other nonunique functions that are not revealed in the deficient mice because of compensatory activities of other cytokines.
These may be detected in future studies of multiply deficient mice.
6. Myeloid Leukemia
Acute and chronic myeloid leukemias (AMLs and CMLs) are clonal diseases of bone marrow cells in which the predominant cell types are granulocytes and macrophages, although leukemic cells of other lineages are also found. There are potentially two ways in which CSFs could be involved in leukemia: They may be required for the proliferation of the leukemic cells, and they may cause differentiation and clonal extinction of the leukemia. In semisolid agar cultures, CML cells will produce colonies of normal appearance containing granulocytes, macrophages, and eosinophils. The colony growth is dependent on CSF stimulation, and all four CSFs are active. In contrast, AML cells produce small clones with little maturation, and growth is very variable from patient to patient. The cells are responsive to CSFs to a variable extent, and in a proportion of patients (<30%) there is colony growth in the absence of CSFs. The colonies do not contain clonogenic cells that are able to form further colonies in secondary cultures. The leukemic stem cells have not been identified, and their dependence on CSFs is unclear. Whether autocrine production of CSFs by leukemic cells is important is also unknown, with the exception of leukemic monocytes, which produce CSFs like their normal counterparts. Lopez and colleagues have produced a GM-CSF antagonist that induced remission in a murine model of juvenile myelomonocytic leukemia (14). Although there are some leukemic cell lines that differentiate and cease proliferation in response to CSFs in vitro, many cell lines are not suppressed by CSFs. There may be defects in the signal transduction pathways that prevent differentiation. In a small number of patients with congenital neutropenia who develop AML, a mutation in the G-CSF receptor expressed by the leukemic cells has been described that prevents differentiation in response to G-CSF (15). However, it is not known whether this mutation contributed to the development of AML.
7. Clinical Use of CSFs
The use of chemotherapy in cancer treatment is limited by its toxicity, primarily neutropenia. Both G-CSF and GM-CSF have been tested extensively in clinical trials and shown to reduce neutropenia, allowing the intensification of chemotherapy doses. Clinical trials have not yet shown, however, whether dose intensification improves survival. G-CSF has fewer side effects and is more effective at elevating neutrophil levels than GM-CSF and thus is usually the CSF of choice, but GM-CSF may be more effective in reducing infectious complications (16). In high-dose chemotherapy followed by autologous bone marrow transplantation, both CSFs reduced the time taken for recovery to ≥500 cells/mm3 by about one week and, in some trials, reduced the duration of hospital stay. G-CSF (and GM-CSF) treatment increased the levels of circulating progenitor and stem cells so that G-CSF-mobilized peripheral blood progenitor cells are now used instead of bone marrow cells for reconstitution after high-dose chemotherapy. This treatment results in more rapid recovery than bone marrow transplantation.
Patients with congenital neutropenia or idiopathic chronic neutropenia respond to G-CSF treatment with elevation of neutrophil levels, resulting in fewer infections and less time in hospital. A few of these patients (3% in Ref. 17) develop myelodysplasia or leukemia, but at present there is no evidence whether G-CSF increases the frequency of these cases. Despite initial concerns that treatment of leukemia patients with CSFs might exacerbate the disease, there is no evidence that this occurs. Neutrophil recovery has occurred more rapidly in leukemia patients receiving G-CSF or GM-CSF after chemotherapy, but reduction in duration of hospital stay was not usually observed.
There have been fewer clinical trials with M-CSF and IL-3. M-CSF appears to improve recovery from fungal infections and is approved in Japan for use in allogeneic transplantation, induction therapy of AML, and dose-intensive therapy of ovarian cancer. IL-3 given after chemotherapy reduced the recovery time from leukopenia and elevated the levels of neutrophils, monocytes, and eosinophils. In the future, it is likely that combinations of cytokines will be tested.
References
1. Y. Yonemura, H. Ku, S. D. Lyman, and M. Ogawa (1997) Blood 89, 1915–1921.
2.G. P. Solar, W. G. Kerr, F. C. Zeigler, D. Hess, C. Donahue, F. J. de Sauvage, and D. L. Eaton (1998) Blood 92, 4–10.
3. J. F. Bazan (1990) Immunol. Today 11, 350–354.
4. Y. Feng, B. K. Klein, and C. A. McWherter (1996) J. Mol. Biol. 259, 524–541.
5. M. Baccarini and E. R. Stanley (1990) In Growth Factors, Differentiation Factors, and Cytokines (A. Habenicht, ed.), Springer-Verlag, Berlin, pp. 188–200.
6. F. H. M. Cluitmans, B. H. J. Esendam, J. E. Landegent, R. Willemze, and J. H. F. Falkenburg (1995) Blood 85, 2038–2044.
7. T. Hara and A. Miyajima (1996) Stem Cells 14, 605–618.
8. B. R. Avalos (1996) Blood 88, 761–777.
9. L. R. Rohrschneider, R. P. Bourette, M. N. Lioubin, P. A. Algate, G. M. Myles, and K. Carlberg (1997) Mol. Reprod. Dev. 46, 96–103 (Abstract).
10. A. W. Roberts, M. Zaiss, A. W. Boyd, and N. A. Nicola (1997) Exp. Hematol. 25, 289–305.
11. W. J. McKinstry, C. Li, J. E. J. Rasko, N. A. Nicola, G. R. Johnson, and D. Metcalf (1997( Blood 89, 65–71.
12. C. S. Lanz, J. Boesiger, C. H. Song, N. Mach, T. Kobayashi, R. C. Mulligan, Y. Nawa, G. Dranoff, and S. J. Galli (1998) Nature 392, 90–93.
13. N. Mach, C. S. Lanz, S. J. Galli, G. Reznikoff, M. Mihm, C. Small, R. Granstein, S. Beissert, M. Sadelain, R. C. Mulligan, and G. Dranoff (1998) Blood 91, 778–783.
14. P. O. Iversen, I. D. Lewis, S. Turczynowicz, H. Hasle, C. Niemeyer, K. Schmiegelow, S. Bastiras, A. Biondi, T. P. Hughes, and A. F. Lopez (1997) Blood 90, 4910–4917.
15. F. Dong, R. K. Brynes, R. K. Tidow, K. Welte, B. Lowenberg, and I. P. Touw (1995) N. Engl. J. Med. 333, 487–493.
16. J. Nemunaitis (1997) Drugs 54, 709–729.
17. D. C. Dale, M. A. Bonilla, M. W. Davis, A. M. Nakanishi, W. P. Hammond, J. Kurtzberg, W. Wang, A. Jakubowski, E. Winton, P. Lalezari, W. Robinson, J. A. Glaspy, S. Emerson, J. Gabrilove, M. Vincent, and L. A. Boxer (1993) Blood 81, 2496–2502.
18. D. Metcalf and N. A. Nicola (1995) The Hemopoietic Colony-Stimulating Factors. From Biology to Clinical Applications, Cambridge University Press, Cambridge, England.
19. R. Callard and A. Gearing (1994) The Cytokine Facts Book, Academic Press, London.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|