


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 6-11-2020
Date: 5-5-2021
Date: 23-4-2021
|
RecA Triggers the SOS System
KEY CONCEPTS
- Damage to DNA causes RecA to trigger the SOS response, which consists of genes coding for many repair enzymes.
- RecA activates the autocleavage activity of LexA.
- LexA represses the SOS system; its autocleavage activates those genes.
When cells respond to DNA damage, the actual repair of the lesion is only one part of the overall response. Eukaryotic cells also engage in two other key types of activities when damage is detected: (1) activation of checkpoints to arrest the cell cycle until the damage is repaired (see the chapter titled Replication Is Connected to the Cell Cycle), and (2) induction of a suite of transcriptional changes that facilitate the damage response (such as production of repair enzymes).
Bacteria also engage in a more global response to damage than just the repair event, known as the SOS response. This response depends on the recombination protein RecA, discussed elsewhere in this chapter. RecA’s role in recombination-repair is only one of its activities. This extraordinary protein also has another quite distinct function: It can be activated by many treatments that damage DNA or inhibit replication in E. coli. This causes it to trigger the SOS response, a complex series of phenotypic changes that involves the expression of many genes whose products include repair functions.
These dual activities of the RecA protein make it difficult to know whether a deficiency in repair in recA mutant cells is due to loss of the DNA strand–exchange function of RecA or to some other function whose induction depends on the protease activity.
The inducing damage can take the form of ultraviolet irradiation (the most studied case) or can be caused by crosslinking or alkylating agents. Inhibition of replication by any of several means—including deprivation of thymine, addition of drugs, or mutations in several of the dna genes—has the same effect.
The response takes the form of increased capacity to repair damaged DNA, which is achieved by inducing synthesis of the components of both the long-patch excision repair system and the
Rec recombination-repair pathways. In addition, cell division is inhibited. Lysogenic prophages may be induced.
The initial event in the response is the activation of RecA by the damaging treatment. We do not know very much about the relationship between the damaging event and the sudden change in RecA activity. A variety of damaging events can induce the SOS response; thus current work focuses on the idea that RecA is activated by some common intermediate in DNA metabolism.
The inducing signal could consist of a small molecule released from DNA, or it might be some structure formed in the DNA itself. In vitro, the activation of RecA requires the presence of singlestranded DNA and ATP. Thus, the activating signal could be the presence of a single-stranded region at a site of damage.
Whatever form the signal takes, its interaction with RecA is rapid: The SOS response occurs within a few minutes of the damaging treatment.
Activation of RecA causes proteolytic cleavage of the product of the lexA gene. LexA is a small (22 kD) protein that is relatively stable in untreated cells, where it functions as a repressor at many operons. The cleavage reaction is unusual: LexA has a latent protease activity that is activated by RecA. When RecA is activated, it causes LexA to undertake an autocatalytic cleavage; this inactivates the LexA repressor function and coordinately induces all the operons to which it was bound. The pathway is illustrated in FIGURE 1.
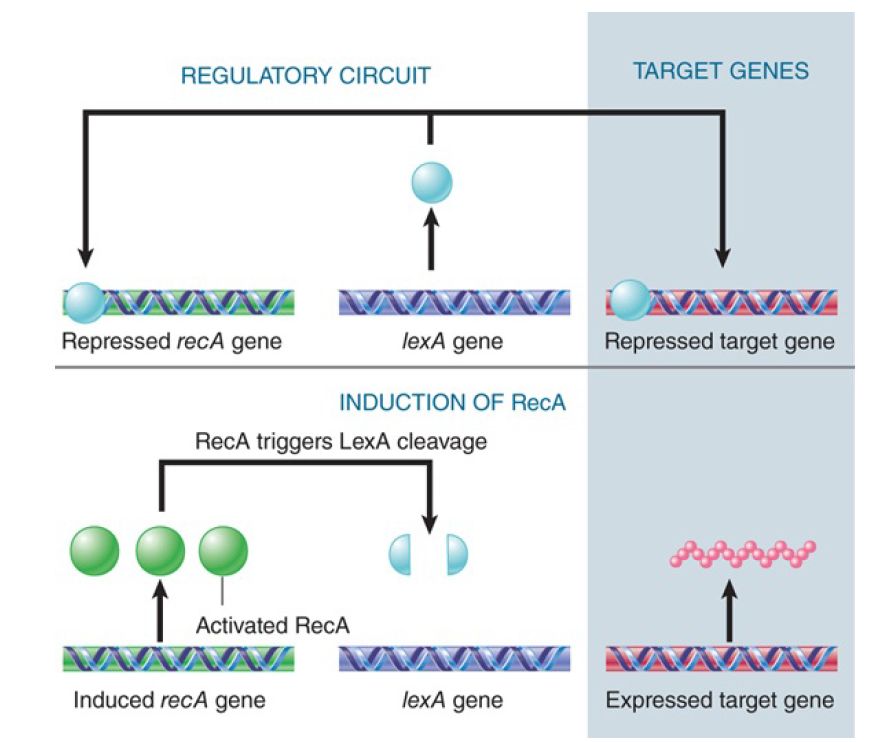
FIGURE 1. The LexA protein represses many genes, includingthe repair genes recA and lexA. Activation of RecA leads to proteolytic cleavage of LexA and induces all of these genes.
The target genes for LexA repression include many with repair functions. Some of these SOS genes are active only in treated cells; others are active in untreated cells, but the level of expression is increased by cleavage of LexA. In the case of uvrB, which is a component of the excision repair system, the gene has two promoters: One functions independently of LexA; the other is subject to its control. Thus, after cleavage of LexA, the gene can be expressed from the second promoter as well as from the first.
LexA represses its target genes by binding to a 20-bp stretch of DNA called an SOS box, which includes a consensus sequence with eight absolutely conserved positions. As is common with other operators, the SOS boxes overlap with the respective promoters. At the lexA locus—the subject of autogenous repression—there are two adjacent SOS boxes.
RecA and LexA are mutual targets in the SOS circuit: RecA triggers cleavage of LexA, which represses recA and itself. The SOS response therefore causes amplification of both the RecA protein and the LexA repressor. The results are not so contradictory as might at first appear.
The increase in expression of RecA protein is necessary (presumably) for its direct role in the recombination-repair pathways. On induction, the level of RecA is increased from its basal level of about 1,200 molecules per cell by up to 50 times.
The high level in induced cells means there is sufficient RecA to ensure that all the LexA protein is cleaved. This should prevent LexA from reestablishing repression of the target genes.
The main importance of this circuit for the cell, however, lies in the cell’s ability to return rapidly to normalcy. When the inducing signal is removed, the RecA protein loses the ability to destabilize LexA.
At this moment, the lexA gene is being expressed at a high level; in the absence of activated RecA, the LexA protein rapidly accumulates in the uncleaved form and turns off the SOS genes. This explains why the SOS response is freely reversible. RecA also triggers cleavage of other cellular targets, sometimes with more direct consequences. The UmuD protein is cleaved when RecA is activated; the cleavage event activates UmuD and the error-prone repair system. The current model for the reaction is that the UmuD UmuC complex binds to a RecA filament near a site of damage, RecA activates the complex by cleaving UmuD to generate UmuD′, and the complex then synthesizes a stretch of DNA to replace the damaged material.
Activation of RecA also causes cleavage of some other repressor proteins, including those of several prophages. Among these is the lambda repressor (with which the protease activity was
discovered). This explains why lambda is induced by ultraviolet irradiation: The lysogenic repressor is cleaved, releasing the phage to enter the lytic cycle.
This reaction is not a cellular SOS response, but instead represents recognition by the prophage that the cell is in trouble. Survival is then best assured by entering the lytic cycle to generate progeny phages. In this sense, prophage induction is piggybacking onto the cellular system by responding to the same indicator (activation of RecA).
The two activities of RecA are relatively independent. The recA441 mutation allows the SOS response to occur without inducing treatment, probably because RecA remains spontaneously in the activated state. Other mutations abolish the ability to be activated.
Neither type of mutation affects the ability of RecA to handle DNA. The reverse type of mutation, inactivating the recombination function but leaving intact the ability to induce the SOS response, would be useful in disentangling the direct and indirect effects of RecA in the repair pathways.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|