


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 23-4-2021
Date: 3-4-2021
Date: 29-4-2021
|
The Weston standard cell
Most electrochemical cells produce about 1.2 V to 1.8 V of electric potential. Different types vary slightly. A mercury cell has a voltage that is a little bit less than that of a zinc-carbon or alkaline cell. The voltage of a cell can also be affected by variables in the manufacturing process. Normally, this is not significant. Most consumer type dry cells can be assumed to produce 1.5 Vdc.
There are certain types of cells whose voltages are predictable and exact. These are called standard cells. One example of a standard cell is the Weston cell. It produces 1.018 V at room temperature. This cell uses a solution of cadmium sulfate. The positive electrode is made from mercury sulfate, and the negative electrode is made using mercury and cadmium. The whole device is set up in a container as shown in Fig. 1. When properly constructed and used at room temperature, the voltage of the Weston standard cell is always the same, and this allows it to be used as a dc voltage standard.
There are other kinds of standard cells, but the Weston cell is the most common.
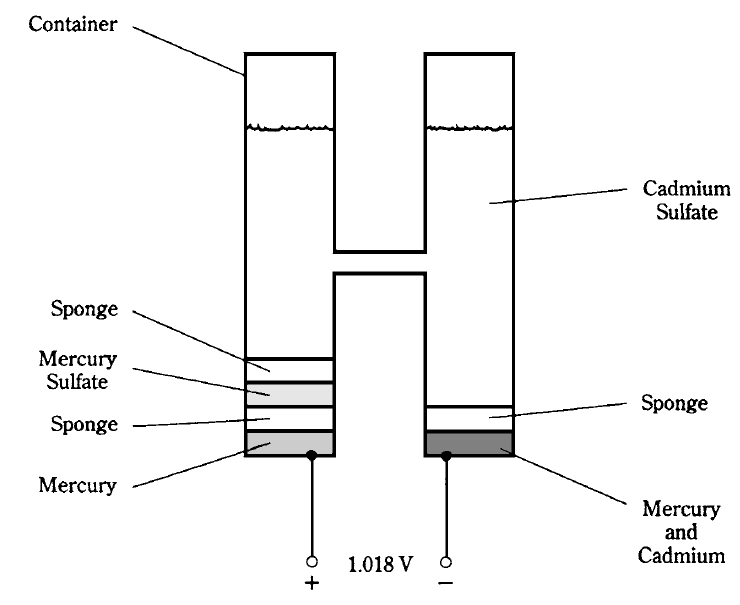
Fig. 1: A Weston standard cell.



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|