
آخر المواضيع المضافة


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 17-9-2020
Date: 31-12-2020
Date: 17-9-2020
|
Pauli Exclusion Principle
But why couldn’t the third lithium electron fall down to the low energy E1 pattern? In 1925, two separate ideas provided the explanation. Wolfgang Pauli proposed that no two electrons were allowed to be in exactly the same state. This is known as the Pauli exclusion principle. But the exclusion principle seems to go too far, because in helium, both electrons are in the same E1 ,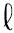 = 0 standing wave pattern. If you cannot have two electrons in exactly the same state in an atom, then something must be different about the two electrons in helium.
= 0 standing wave pattern. If you cannot have two electrons in exactly the same state in an atom, then something must be different about the two electrons in helium.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
أصواتٌ قرآنية واعدة .. أكثر من 80 برعماً يشارك في المحفل القرآني الرمضاني بالصحن الحيدري الشريف
|
|
|