


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 1-9-2020
Date: 24-2-2016
Date: 27-8-2020
|
Basic spectrometry: Natural line width
The transitions from one energy level to another are not instantaneous and, as a consequence, the radiation absorbed or emitted does not occur at a unique frequency. The natural spread of any generated line depends on the ‘lifetimes’ of the excited states of the particular atom. A short lifetime produces narrow lines—as the lifetime increases the natural spread of the line increases.
If the Heisenberg uncertainty principle is applied, the energy of a given state cannot be assigned more specifically than
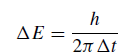
where Δt is the lifetime of the state. Consequently, any assembly of atoms produces a spectral line in emission or absorption with a spread in frequency such that
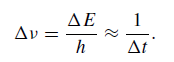
A typical excited atomic state may have a lifetime ∼10−8 s and, in the visible spectral domain (∼550 nm), this provides a wavelength spread ∼1·6 × 10−5 nm or 0·016 mA˚ . (In order to check the calculation it will be remembered that Δλ = λ2/c × Δν.)
In most physical situations, practical determination of natural emission widths is compromised by a variety of physical causes affecting the behaviour of the radiating atoms. Certainly this is the case in the general astrophysical environments as highlighted in the next three subsections which serve as examples of the importance of recording the details of spectral line profiles.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|