

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
IUPAC Rules for Nomenclature of substituted Cycloalkanes
المؤلف:
LibreTexts Project
المصدر:
................
الجزء والصفحة:
.................
26-1-2020
2163
IUPAC Rules for Nomenclature of substituted Cycloalkanes
The naming of substituted cycloalkanes follows the same basic steps used in naming alkanes.
- Determine the parent chain.
- Number the substituents of the ring so that the sum of the numbers is the lowest possible.
- Name the substituents and place them in alphabetical order.
More specific rules for naming substituted cycloalkanes with examples are given below.
- Determine the cycloalkane to use as the parent. If there is an alkyl straight chain that has a greater number of carbons than the cycloalkane, then the alkyl chain must be used as the primary parent chain. Cycloalkanes substituents have an ending "-yl". If there are two cycloalkanes in the molecule, use the cycloalkane with the higher number of carbons as the parent.
Example 1.1
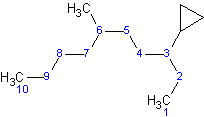
The longest straight chain contains 10 carbons, compared with cyclopropane, which only contains 3 carbons. The parent chain in this molecule is decane and cyclopropane is a substituent, . The name of this molecule is 3-cyclopropyl-3,10-dimethyldecane.
2) When there is only one substituent on the ring, the ring carbon attached to the substituent is automatically carbon #1. Indicating the number of the carbon with the substituent in the name is optional.
Example 1.2
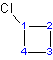 |
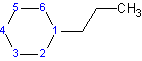 |
| 1-chlorocyclobutane or cholorocyclobutane | 1-propylcyclohexane or propylcyclohexane |
If there are multiple substituents on the ring, number the carbons of the cycloalkane so that the carbons with substituents have the lowest possible number. A carbon with multiple substituents should have a lower number than a carbon with only one substituent or functional group. One way to make sure that the lowest number possible is assigned is to number the carbons so that when the numbers corresponding to the substituents are added, their sum is the lowest possible.
Example 1.3
.gif?revision=1&size=bestfit&width=171&height=129) (1+3=4) NOT
(1+3=4) NOT 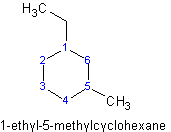.gif?revision=1&size=bestfit&width=171&height=132) (1+5=6)
(1+5=6)
3) When naming the cycloalkane, the substituents must be placed in alphabetical order. Remember the prefixes di-, tri-, etc. , are not used for alphabetization.
Example 1.4
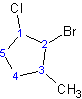
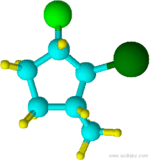
2-bromo-1-chloro-3-methylcyclopentane
Notice that "f" of fluoro alphabetically precedes the "m" of methyl.
Example 1.5
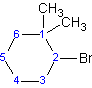
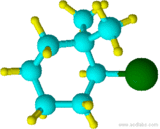
(2-bromo-1,1-dimethylcyclohexane)
Although "di" alphabetically precedes "f", "di" is not used in determining the alphabetical order.
Example 1.6

(2-fluoro-1,1,-dimethylcyclohexane NOT 1,1-dimethyl-2-fluorocyclohexane)
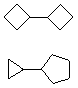
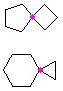
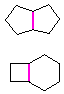
Hydrocarbons having more than one ring are common, and are referred to as bicyclic (two rings), tricyclic (three rings) and in general, polycyclic compounds. The molecular formulas of such compounds have H/C ratios that decrease with the number of rings. In general, for a hydrocarbon composed of n carbon atoms associated with m rings the formula is: CnH(2n + 2 - 2m). The structural relationship of rings in a polycyclic compound can vary. They may be separate and independent, or they may share one or two common atoms. Some examples of these possible arrangements are shown in the following table.
Table 1.1: Examples of Isomeric Bicycloalkanes
| Isolated Rings | Spiro Rings | Fused Rings | Bridged Rings |
|---|---|---|---|
| No common atoms | One common atom | One common bond | Two common atoms |
 |
 |
 |
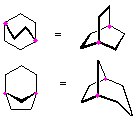 |
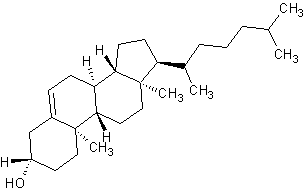
Polycyclic compounds, like cholesterol shown below, are biologically important and typically have common names accepted by IUPAC. However, the common names do not generally follow the basic IUPAC nomenclature rules, and will not be covered here.
|
|
|
Cholesterol (polycyclic) |
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















