


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 10-8-2018
Date: 2-1-2020
Date: 7-8-2019
|
Arenes have absorption bands in the 650-900 cm−1 region due to bending of the C–H bond out of the plane of the ring. The exact placement of these absorptions can indicate the pattern of substitution on a benzene ring. However, this is beyond the scope of introductory organic chemistry. Arenes also possess a characteristic absorption at about 3030-3100 cm−1 as a result of the aromatic C–H stretch. It is somewhat higher than the alkyl C–H stretch (2850–2960 cm−1), but falls in the same region as olefinic compounds. Two bands (1500 and 1660 cm−1) caused by C=C in plane vibrations are the most useful for characterization as they are intense and are likely observed.
In aromatic compounds, each band in the spectrum can be assigned:
Note that this is at slightly higher frequency than is the –C–H stretch in alkanes. This is a very useful tool for interpreting IR spectra. Only alkenes and aromatics show a C–H stretch slightly higher than 3000 cm-1.
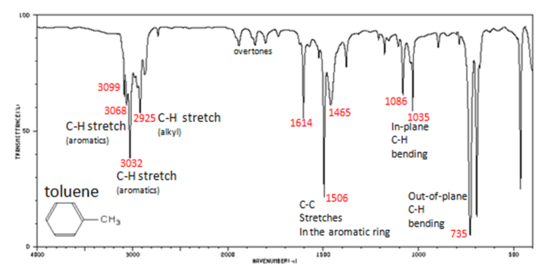
Figure 1. Infrared Spectrum of Toluene



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|