
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-9-2018
Date: 30-8-2019
Date: 27-11-2019
|
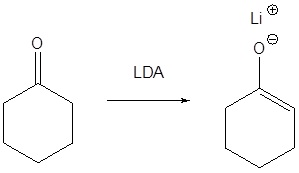
For alkylation reactions of enolate anions to be useful, these intermediates must be generated in high concentration in the absence of other strong nucleophiles and bases. The aqueous base conditions used for the aldol condensation are not suitable because the enolate anions of simple carbonyl compounds are formed in very low concentration, and hydroxide or alkoxide bases induce competing SN2 and E2 reactions of alkyl halides. It is necessary, therefore, to achieve complete conversion of aldehyde or ketone reactants to their enolate conjugate bases by treatment with a very strong base (pKa > 25) in a non-hydroxylic solvent before any alkyl halides are added to the reaction system. Some bases that have been used for enolate anion formation are: NaH (sodium hydride, pKa > 45), NaNH2 (sodium amide, pKa = 34), and LiN[CH(CH3)2]2 (lithium diisopropylamide, LDA, pKa 36). Ether solvents like tetrahydrofuran (THF) are commonly used for enolate anion formation. With the exception of sodium hydride and sodium amide, most of these bases are soluble in THF. Certain other strong bases, such as alkyl lithium and Grignard reagents, cannot be used to make enolate anions because they rapidly and irreversibly add to carbonyl groups. Nevertheless, these very strong bases are useful in making soluble amide bases. In the preparation of lithium diisopropylamide (LDA), for example, the only other product is the gaseous alkane butane.
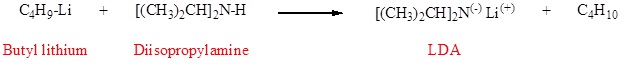

Because of its solubility in THF, LDA is a widely used base for enolate anion formation. In this application, one equivalent of diisopropylamine is produced along with the lithium enolate, but this normally does not interfere with the enolate reactions and is easily removed from the products by washing with aqueous acid. Although the reaction of carbonyl compounds with sodium hydride is heterogeneous and slow, sodium enolates are formed with the loss of hydrogen, and no other organic compounds are produced.
The presence of these overlapping p orbitals gives α
hydrogens (Hydrogens on carbons adjacent to carbonyls) special properties. In particular, α hydrogens are weakly acidic because the conjugate base, called an enolate, is stabilized though conjugation with the π orbitals of the carbonyl. The effect of the carbonyl is seen when comparing the pKa for the α
hydrogens of aldehydes (~16-18), ketones (~19-21), and esters (~23-25) to the pKa of an alkane (~50).
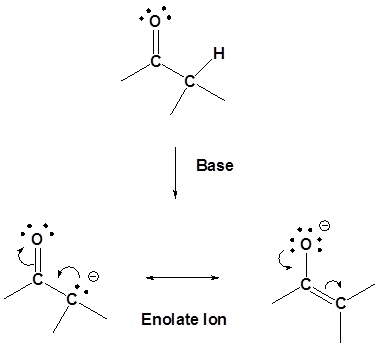
Of the two resonance structures of the enolate ion the one which places the negative charge on the oxygen is the most stable. This is because the negative change will be better stabilized by the greater electronegativity of the oxygen.
| Functional Group | Structure | pKa |
|---|---|---|
| carboxylic acid | HO–(C=O)R | 5 |
| nitro | RCH2–NO2 | 9 |
| β-diketone * | R(O=C)–CH2–(C=O)R | 9 |
| β-ketoester * | R(O=C)–CH2–(C=O)OR | 11 |
| β-diester * | RO(O=C)–CH2–(C=O)OR | 13 |
| amide | RNH–(C=O)R | 15 |
| alcohol | RCH2–OH | 16 |
| aldehyde | RCH2–(C=O)H | 17 |
| ketone | RCH2–(C=O)R | 20 |
| thioester | RCH2–(C=O)SR | 21 |
| ester | RCH2–(C=O)OR | 25 |
| nitrile | RCH2–C≡N | 25 |
| sulfone | RCH2–SO2R | 25 |
| amide | RCH2–(C=O)N(CH3)2 | 30 |
| alkane | CH3–R | 50 |
* Note methylene groups bridging between two electron withdrawing groups are more acidic than alpha protons next to only one carbonyl group.

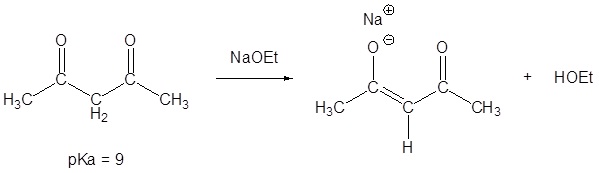
If the formed enolate is stabilized by more than one carbonyl it is possible to use a weaker base such as sodium ethoxide.
NaOCH2CH3 = Na+ -OCH2CH3 = NaOEt
Because of the acidity of α hydrogens, carbonyls undergo keto-enol tautomerism. Tautomers are rapidly interconverted constitutional isomers, usually distinguished by a different bonding location for a labile hydrogen atom and a differently located double bond. The equilibrium between tautomers is not only rapid under normal conditions, but it often strongly favors one of the isomers (acetone, for example, is 99.999% keto tautomer). Even in such one-sided equilibria, evidence for the presence of the minor tautomer comes from the chemical behavior of the compound. Tautomeric equilibria are catalyzed by traces of acids or bases that are generally present in most chemical samples.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
وفد كلية الزراعة في جامعة كربلاء يشيد بمشروع الحزام الأخضر
|
|
|