

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Hydroxyl Acidity
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
11-9-2018
1618
Hydroxyl Acidity
The carboxyl group is an outstanding example of the interaction of two functional groups (hydroxyl and carbonyl) when they are bonded together. One manifestation of this interaction is the enhanced acidity of the carboxyl group relative to an isolated hydroxyl group (more than ten powers of ten). This increase in acidity was explained earlier by resonance stabilization of the carboxylate anion conjugate base. As shown below (first equation), this results in negative charge delocalization over two oxygens as compared with full charge localization on an alkoxide oxygen.
A carbon-carbon double bond can conjugatively link hydroxyl and carbonyl groups so that the corresponding alkoxide base is similarly stabilized by charge delocalization. The second equation illustrates this vinylagous relationship, and a green box identifies the double bond that establishes the vinylagous link. A third resonance contributor, which has the negative charge on the central carbon atom, has been omitted from this drawing, but is an important factor in alkylation reactions of beta-dicarbonyl compounds.
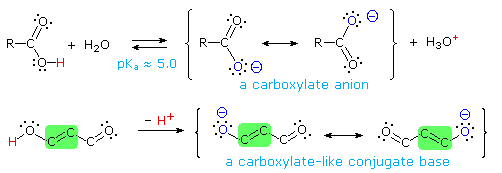
Some examples of vinylagous acids are shown below, together with their pKa values. The first compound, the enol of 1,3-cyclohexanedione, fits the general example outlined above, and has an acidity comparable to acetic acid. The second example (tropolone) is triply vinylagous. Although it is less acidic than a carboxylic acid, it is much more acidic than an alcohol and even a thousand times greater than phenol.
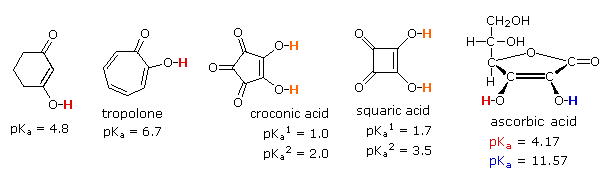
The third and fourth examples listed are extraordinary cases in which vinylagous activation enhances the acidity of two hydroxyl functions in the same molecule. Because of symmetry, it is not possible to identify which is more acidic, but the first pKa ranks these acids stronger than phosphoric acid. The high acidity of the second hydroxyl function is even more surprising, and undoubtedly reflects the fact that both conjugate bases are stabilized by charge delocalization over a different set of oxygen atoms. The case of ascorbic acid, commonly known as Vitamin C provides the last example. Here it should be clear that the beta-hydroxyl group (the red hydrogen) is vinylagously activated by the carbonyl function, and is a stronger acid than acetic acid. The alpha-hydroxyl group (blue hydrogen) is part of an enol, and is therefore expected to have an acidity similar to phenol.
If the hydroxyl group of a vinylagous acid is replaced by an amino group or an alkoxyl group the resulting compounds may be classified as vinylagous amides and esters respectively. The following general formulas illustrate these terms. As expected from the electron interactions shown by the resonance formulas, the properties of such compounds are similar to amides and esters. The basicity of a vinylagous amide, for example, is much less than that of an enamine.
|
Vinylagous Amide |
R2N–C=C–C=O |
 |
R2N(+)=C–C=C–O(–) |
|---|---|---|---|
|
Vinylagous Ester |
RO–C=C–C=O |
 |
RO(+)=C–C=C–O(–) |
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















