
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 1-9-2019
Date: 12-9-2019
Date: 23-8-2019
|
Resonance Structures are a representation of a Resonance Hybrid, which is the combination of all resonance structures. Though the Formal Charge closest to zero is the most accepted structure, in reality the correct Lewis structure is actually a combination of all the resonance structures (and hence is not solely describe as one).
Example 1
Consider the carbonate ion: CO32-
SOLUTION
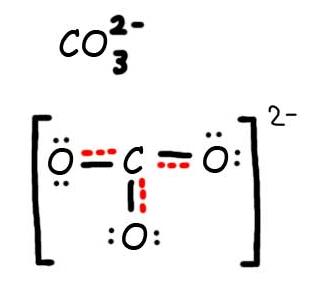
Step 1: Draw the Lewis Structure & Resonance.
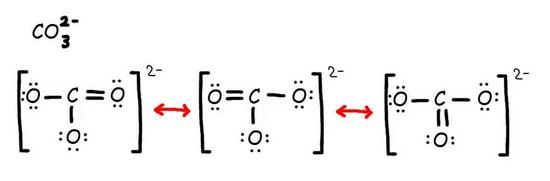
Step 2: Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed.
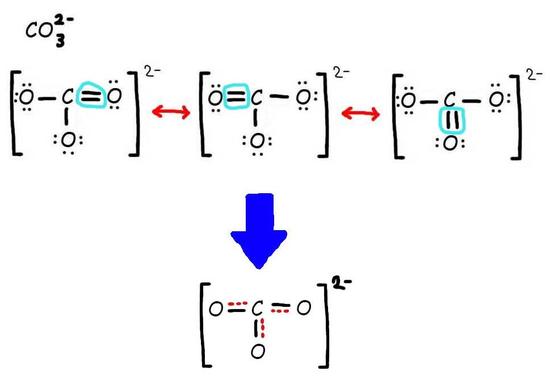
Step 3: Add only the lone pairs found on ALL resonance structures.
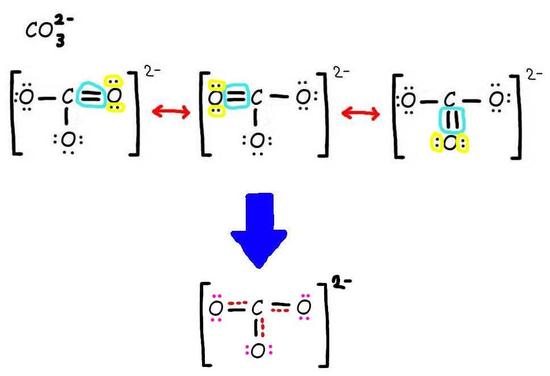
The bottom is the finished resonance hybrid for CO32-.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|