


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 1-9-2019
Date: 1-10-2019
Date: 2-12-2019
|
* Notes
This section contains little that is new. If you mastered the IUPAC nomenclature of alkanes, you should have little difficulty in naming alkyl halides. Notice that when a group such as CH2Br must be regarded as a substituent, rather than as part of the main chain, we may use terms such as bromomethyl.
You will find it easier to understand the reactions of the alkyl halides if you keep the polarity of the C−X
bond fixed permanently in your mind (see ”The Polar C− X Bond” shown in the reading below).
Alkyl halides are also known as haloalkanes. This page explains what they are and discusses their physical properties. alkyl halides are compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine or iodine). We will only look at compounds containing one halogen atom. For example:
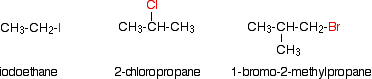
alkyl halides fall into different classes depending on how the halogen atom is positioned on the chain of carbon atoms. There are some chemical differences between the various types.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
مركز الثقافة الأسرية ينظم برنامج (ربيع الثقافة المعرفي) لطالبات ثانوية الزهراء في بغداد
|
|
|