
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-12-2015
Date: 12-5-2017
Date: 11-9-2019
|
Example.1
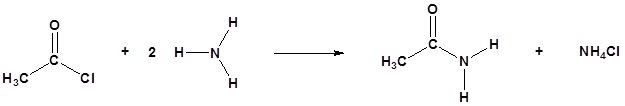
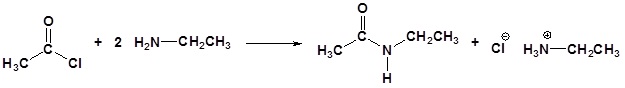
Amine functions seldom serve as leaving groups in nucleophilic substitution or base-catalyzed elimination reactions. Indeed, they are even less effective in this role than are hydroxyl and alkoxyl groups. In the case of alcohols and ethers, a useful technique for enhancing the reactivity of the oxygen function was to modify the leaving group (OH(–) or OR(–)) to improve its stability as an anion (or equivalent). This stability is conveniently estimated from the strength of the corresponding conjugate acids.
As noted earlier, 1º and 2º-amines are much weaker acids than alcohols, so it is not surprising that it is difficult to force the nitrogen function to assume the role of a nucleophilic leaving group. For example, heating an amine with HBr or HI does not normally convert it to the corresponding alkyl halide, as in the case of alcohols and ethers. In this context we note that the acidity of the putative ammonium leaving group is at least ten powers of ten less than that of an analogous oxonium species. The loss of nitrogen from diazonium intermediates is a notable exception in this comparison, due to the extreme stability of this leaving group (the conjugate acid of N2 would be an extraordinarily strong acid).
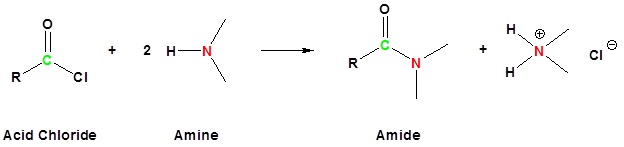
One group of amine derivatives that have proven useful in SN2 and E2 reactions is that composed of the tetraalkyl (4º-) ammonium salts. Most applications involving this class of compounds are eliminations, but a few examples of SN2 substitution have been reported.
C6H5–N(CH3)3(+) Br(–) + R-S(–) Na(+) |
acetone & heat |
R-S-CH3 + C6H5–N(CH3)2 + NaBr |
(CH3)4N(+) OH(–) |
heat |
CH3–OH + (CH3)3N |



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|