


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-9-2019
Date: 28-10-2019
Date: 28-10-2019
|
Catalytic hydrogenation of aromatic rings requires forcing conditions (high heat and hydrogen pressure).

Under milder conditions it is possible to reduce the double-bond of an alkene without reducing the aromatic ring.

Notice in the above equation that H2/Pd does not reduce the keto-carbonyl group. Remember, however, that H2/Pd will reduce a keto-carbonyl group when it is directly attached to an aromatic ring (see equations 4 and 5 under Carbonyl Reductions).
This reduction of the C=O group next to an aromatic ring is an important synthetic tool. Recall the Friedel-Crafts alkylation from Section 16.3. When attaching larger alkyl groups to arenes there is a possibility of rearrangement of the alkyl group structure.
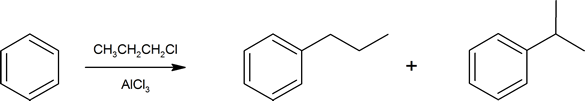
To generate the target compound (in this case n‑propylbenzene) in a more controlled fashion, one can simply use the equivalent Friedel-Crafts acylation and then reduce the keto-carbonyl group next to the ring as a final step.




|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
المجمع العلمي يقيم دورة تطويرية عن أساليب التدريس ويختتم أخرى تخص أحكام التلاوة
|
|
|