


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-1-2018
Date: 1-4-2019
Date: 28-12-2018
|
Sulfur can be mined by the Frasch process. This process has made sulfur a high purity (up to 99.9 percent pure) chemical commodity in large quantities. Most sulfur-containing minerals are metal sulfides, and the best known is perhaps pyrite (FeS2, known as fools gold because of its golden color). The most common sulfate containing mineral is gypsum, CaSO4⋅2H2O, also known as plaster of Paris.
The Frasch process is based on the fact that sulfur has a comparatively low melting point. The process forces (99.5% pure) sulfur out by using hot water and air. In this process, superheated water is forced down the outermost of three concentric pipes. Compressed air is pumped down the center tube, and a mixture of elemental sulfur, hot water, and air comes up the middle pipe. Sulfur is melted with superheated water (at 170 °C under high pressure) and forced to the surface of the earth as a slurry.
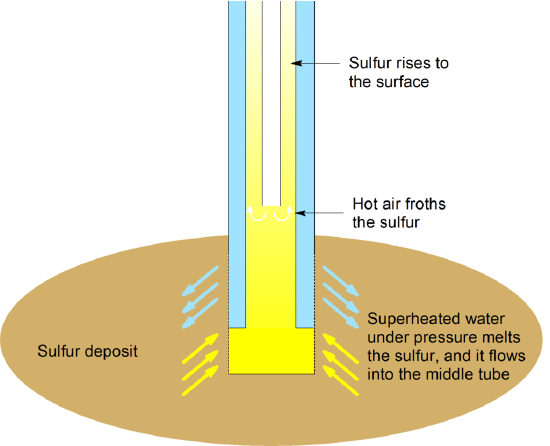
Figure 1.1: Pictorial representation of the Frasch process. Adapted from Wolfgang Nehb, Karel Vydra (2005), "Sulfur", Ullmann’s Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH. Image used with permission (Public Domain; Rifleman 82)
Sulfur is mostly used for the production of sulfuric acid, H2SO4. Most sulfur mined by Frasch process is used in industry for the manufacture of sulfuric acid. Sulfuric acid, the most abundantly produced chemical in the United States, is manufactured by the contact process. Most (about 70%) of the sulfuric acid produced in the world is used in the fertilizer industry. Sulfuric acid can act as a strong acid, a dehydrating agent, and an oxidizing agent. Its applications use these properties. Sulfur is an essential element of life in sulfur-containing proteins.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|