


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 10-3-2019
Date: 1-1-2018
Date: 13-3-2019
|
Glass is another silicon derivate that is widely utilized by modern day society. If sand, a silica deposit, is mixed with sodium and calcium carbonate at temperatures near 1500 degrees Celsius, when the resulting product cools, glass forms. Glass is a particularly interesting state of silicon. Glass is unique because it represents a solid non-crystalline form of silicon. The tetrahedral silica elements bind together, but in no fundamental pattern behind the bonding. (see Figure 1)
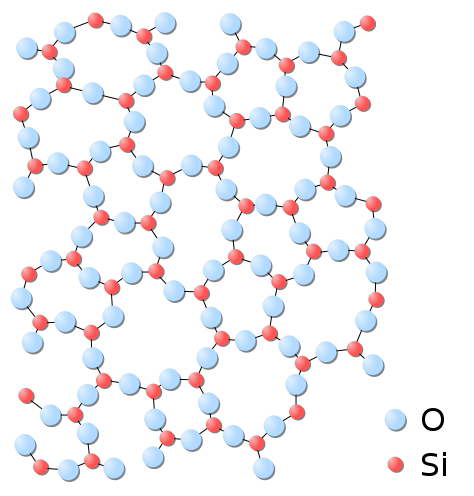
Figure 1: Non-crystalline silica
The end result of this unique chemical structure is the often brittle typically optically transparent material known as glass. This silica complex can be found virtually anywhere human civilization is found.
Glass can be tainted by adding chemical impurities to the basal silica structure. (see Figure 17) The addition of even a little Fe2O3 to pure silica glass gives the resultant mixed glass a distinctive green color.

Non-crystalline silica with unknown impurities Figure 1



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|