


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-12-2020
Date: 23-9-2018
Date: 18-12-2020
|
Reversible or Opposing Reactions
In reversible or opposing reactions the products formed also react to give back the reactants.
Initially, the rate of forward reaction is very large which decreases with passage of time and the rate of backward or reverse reaction is zero which increases with passage of item. A stage is reached when two rates become equal. This situation is called the chemical equilibrium. It is dynamic in nature i.e., all the species are reaching at the rate at which they are being formed. A reaction of this type may be represented as

where kf and kb are the rate constants of the forward and backward reactions respectively.
The overall rate of reaction is given by
Rate of Reaction = Rate of forward reaction – Rate of backward reaction
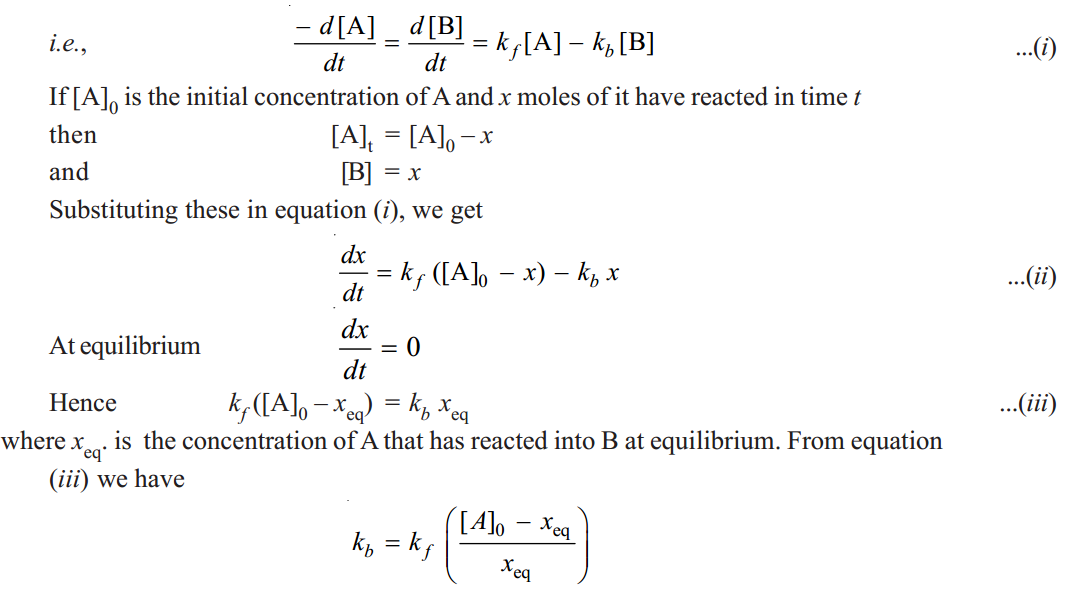
Substituting the value of kb in equation (ii), we get
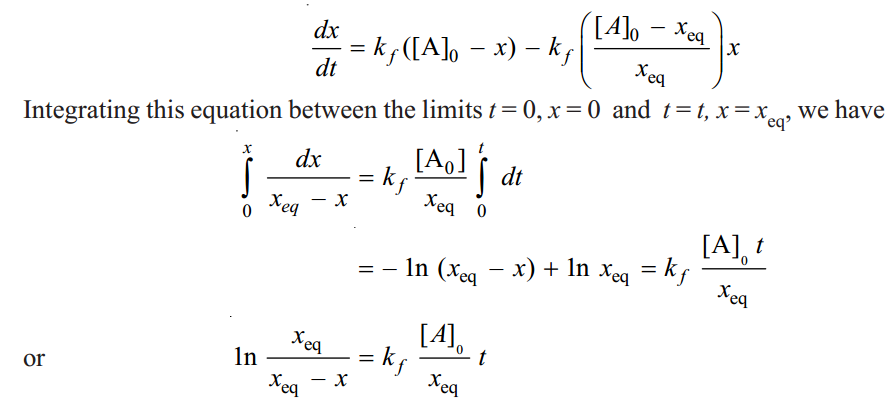
From this equation we can find the value of kf from the quantities [A]0, xeq and x at time t. All these quantities can be measured easily. From the value of kf the value of kb can be calculated by using the relation.
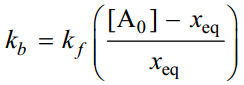



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مستشفى العتبة العباسية الميداني في سوريا يقدّم خدماته لنحو 1500 نازح لبناني يوميًا
|
|
|