


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-1-2020
Date: 13-1-2020
Date: 23-5-2017
|
As with alkenes, the addition of water to alkynes requires a strong acid, usually sulfuric acid, and is facilitated by mercuric sulfate. However, unlike the additions to double bonds which give alcohol products, addition of water to alkynes gives ketone products ( except for acetylene which yields acetaldehyde ). The explanation for this deviation lies in enol-keto tautomerization, illustrated by the following equation. The initial product from the addition of water to an alkyne is an enol (a compound having a hydroxyl substituent attached to a double-bond), and this immediately rearranges to the more stable keto tautomer.
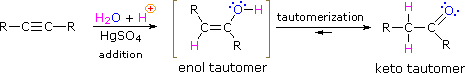
Tautomers are defined as rapidly interconverted constitutional isomers, usually distinguished by a different bonding location for a labile hydrogen atom (colored red here) and a differently located double bond. The equilibrium between tautomers is not only rapid under normal conditions, but it often strongly favors one of the isomers ( acetone, for example, is 99.999% keto tautomer ). Even in such one-sided equilibria, evidence for the presence of the minor tautomer comes from the chemical behavior of the compound. Tautomeric equilibria are catalyzed by traces of acids or bases that are generally present in most chemical samples. The three examples shown below illustrate these reactions for different substitutions of the triple-bond. The tautomerization step is indicated by a red arrow. For terminal alkynes the addition of water follows the Markovnikov rule, as in the second example below, and the final product ia a methyl ketone ( except for acetylene, shown in the first example ). For internal alkynes ( the triple-bond is within a longer chain ) the addition of water is not regioselective. If the triple-bond is not symmetrically located ( i.e. if R & R' in the third equation are not the same ) two isomeric ketones will be formed.
| HC≡CH + H2O + HgSO4 & H2SO4 ——> [ H2C=CHOH ] ——> H3C-CH=O |
| RC≡CH + H2O + HgSO4 & H2SO4 ——> [ RC(OH)=CH2 ] ——> RC(=O)CH3 |
| RC≡CR' + H2O + HgSO4 & H2SO4 ——> [ RHC=C(OH)R' + RC(OH)=CHR' ] ——> RCH2-C(=O)R' + RC(=O)-CH2R' |
Two factors have an important influence on the enol-keto tautomerizations described here. The first is the potential energy difference between the tautomeric isomers. This factor determines the position of the equilibrium state. The second factor is the activation energy for the interconversion of one tautomer to the other. This factor determines the rate of rearrangement. Since the potential energy or stability of a compound is in large part a function of its covalent bond energies, we can estimate the relative energy of keto and enol tautomers by considering the bonds that are changed in the rearrangement. From the following diagram, we see that only three significant changes occur, and the standard bond energies for those changes are given to the right of the equation. The keto tautomer has a 17.5 kcal/mole advantage in bond energy, so its predominance at equilibrium is expected.
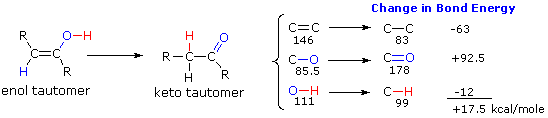
The rapidity with which enol-keto tautomerization occurs suggests that the activation energy for this process is low. We have noted that the rearrangement is acid & base catalyzed, and very careful experiments have shown that interconversion of tautomers is much slower if such catalysts are absent. A striking example of the influence of activation energy on such transformations may be seen in the following hypothetical rearrangement. Here we have substituted a methyl group (colored maroon) for the proton of a conventional tautomerism, and the methyl shifts from oxygen to carbon just as the proton does in going from an enol to a ketone.
H2C=CH-O-CH3 –X–> CH3-CH2-CH=O
The potential energy change for this rearrangement is even more advantageous than for enol-keto tautomerism, being estimated at over 25 kcal/mole from bond energy changes. Despite this thermodynamic driving force, the enol ether described above is completely stable to base treatment, and undergoes rapid acid-catalyzed hydrolysis with loss of methanol, rather than rearrangement. The controlling difference in this case must be a prohibitively high activation energy for the described rearrangement, combined with lower energy alternative reaction paths.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|