


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-11-2019
Date: 2-11-2019
Date: 2-10-2018
|
Addition of a hydride anion to an aldehyde or ketone would produce an alkoxide anion, which on protonation should yield the corresponding alcohol. Aldehydes would give 1º-alcohols (as shown) and ketones would give 2º-alcohols.
RCH=O + H:(–)  RCH2O(–) + H3O(+)
RCH2O(–) + H3O(+)  RCH2OH
RCH2OH
Two practical sources of hydride-like reactivity are the complex metal hydrides lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4). These are both white (or near white) solids, which are prepared from lithium or sodium hydrides by reaction with aluminum or boron halides and esters. Lithium aluminum hydride is by far the most reactive of the two compounds, reacting violently with water, alcohols and other acidic groups with the evolution of hydrogen gas. The following table summarizes some important characteristics of these useful reagents.
|
Reagent |
Preferred Solvents |
Functions Reduced |
Reaction Work-up |
|---|---|---|---|
| Sodium Borohydride NaBH4 |
ethanol; aqueous ethanol 15% NaOH; diglyme avoid strong acids |
aldehydes to 1º-alcohols ketones to 2º-alcohols inert to most other functions |
1) simple neutralization 2) extraction of product |
| Lithium Aluminum Hydride LiAlH4 |
ether; THF avoid alcohols and amines avoid halogenated compounds avoid strong acids |
aldehydes to 1º-alcohols ketones to 2º-alcohols carboxylic acids to 1º-alcohols esters to alcohols epoxides to alcohols nitriles & amides to amines halides & tosylates to alkanes most functions react |
1) careful addition of water 2) remove aluminum salts 3) extraction of product |
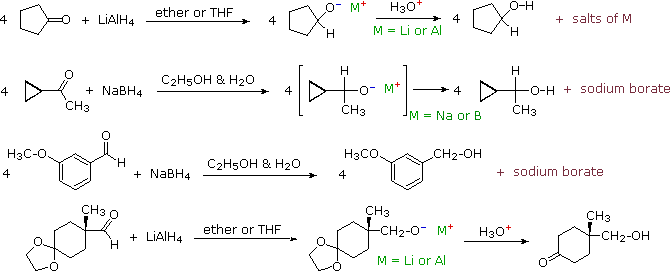
Some examples of aldehyde and ketone reductions, using the reagents described above, are presented in the following diagram. The first three reactions illustrate that all four hydrogens of the complex metal hydrides may function as hydride anion equivalents which bond to the carbonyl carbon atom. In the LiAlH4 reduction, the resulting alkoxide salts are insoluble and need to be hydrolyzed (with care) before the alcohol product can be isolated. In the borohydride reduction the hydroxylic solvent system achieves this hydrolysis automatically. The lithium, sodium, boron and aluminum end up as soluble inorganic salts. The last reaction shows how an acetal derivative may be used to prevent reduction of a carbonyl function (in this case a ketone). Remember, with the exception of epoxides, ethers are generally unreactive with strong bases or nucleophiles. The acid catalyzed hydrolysis of the aluminum salts also effects the removal of the acetal. This equation is typical in not being balanced (i.e. it does not specify the stoichiometry of the reagent).
Reduction of α,β-unsaturated ketones by metal hydride reagents sometimes leads to a saturated alcohol, especially with sodium borohydride. This product is formed by an initial conjugate addition of hydride to the β-carbon atom, followed by ketonization of the enol product and reduction of the resulting saturated ketone (equation 1 below). If the saturated alcohol is the desired product, catalytic hydrogenation prior to (or following) the hydride reduction may be necessary. To avoid reduction of the double bond, cerium(III) chloride is added to the reaction and it is normally carried out below 0 ºC, as shown in equation 2.
| 1) RCH=CHCOR' | + | NaBH4 (aq. alcohol) | ——> | RCH=CHCH(OH)R' | + | RCH2-CH2CH(OH)R' |
| 1,2-addition product | 1,4-addition product | |||||
| 2) RCH=CHCOR' | + | NaBH4 & CeCl3 -15º | ——> | RCH=CHCH(OH)R' | ||
| 1,2-addition product | ||||||
Before leaving this topic it should be noted that diborane, B2H6, a gas that was used in ether solution to prepare alkyl boranes from alkenes, also reduces many carbonyl groups. Consequently, selective reactions with substrates having both functional groups may not be possible. In contrast to the metal hydride reagents, diborane is a relatively electrophilic reagent, as witnessed by its ability to reduce alkenes. This difference also influences the rate of reduction observed for the two aldehydes shown below. The first, 2,2-dimethylpropanal, is less electrophilic than the second, which is activated by the electron withdrawing chlorine substituents.
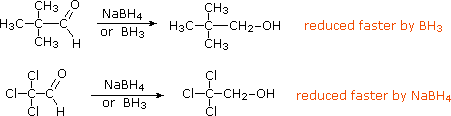



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|