


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 20-7-2018
Date: 20-7-2018
Date: 8-12-2019
|
The configuration properties of D-glucose.
1. Ribose and arabinose (two well known pentoses) both gave erythrose on Ruff degradation. As expected, Kiliani-Fischer synthesis applied to erythrose gave a mixture of ribose and arabinose.
2. Oxidation of erythrose gave an achiral (optically inactive) aldaric acid. This defines the configuration of erythrose.
3. Oxidation of ribose gave an achiral (optically inactive) aldaric acid. This defines the configuration of both ribose and arabinose.
4. Ruff shortening of glucose gave arabinose, and Kiliani-Fischer synthesis applied to arabinose gave a mixture of glucose and mannose.
5. Glucose and mannose are therefore epimers at C-2, a fact confirmed by the common product from their osazone reactions.
6. A pair of structures for these epimers can be written, but which is glucose and which is mannose?
In order to determine which of these epimers was glucose, Fischer made use of the inherent C2 symmetry in the four-carbon dissymmetric core of one epimer (B). This is shown in the following diagram by a red dot where the symmetry axis passes through the projection formula. Because of this symmetry, if the aldehyde and 1º-alcohol functions at the ends of the chain are exchanged, epimer B would be unchanged; whereas A would be converted to a different compound. By clicking on the diagram, the consequences of such an exchange will be displayed.
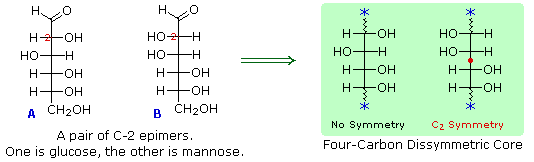
Fischer looked for and discovered a second aldohexose that represented the end group exchange for the epimer lacking the latent C2 symmetry (A). This compound was L-(+)-gulose, and its exchange relationship to D-(+)-glucose was demonstrated by oxidation to a common aldaric acid product. Equations for this operation will be displayed by clicking again on the above diagram. The remaining epimer is therefore mannose.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|