

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The Ortho Effect of benzoic acids
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
12-7-2018
3364
The Ortho Effect of benzoic acids
In general, ortho-substituted are stronger acids than their meta and para isomers, regardless of the nature of the substituent. The ortho effect is large for the nitrobenzoic acids, which show nearly a 20 fold increase in acidity, roughly an 8 fold factor for the halobenzoic acids, and a 2.5 to 3 fold increase for methyl and cyano substituents. The methoxybenzoic acids are exceptional, in that the ortho and meta isomers have nearly identical pKa's (ca. 4.1), presumably due to the exceptional p-π electron donation from oxygen noted above.
Many of the factors that influence the acidity of substituted benzoic acids are summarized in the following diagram. First, although the phenyl group is inductively electron withdrawing, it can donate electrons to a carboxyl group by π-π resonance, as shown in the green shaded box in the upper left. A substituent Y may perturb the balance of these two factors by its inductive influence or by resonance. Two resonance cases, one showing electron withdrawal by a nitro substituent and the other electron donation by a methoxy substituent, are shown to the right of the green box.
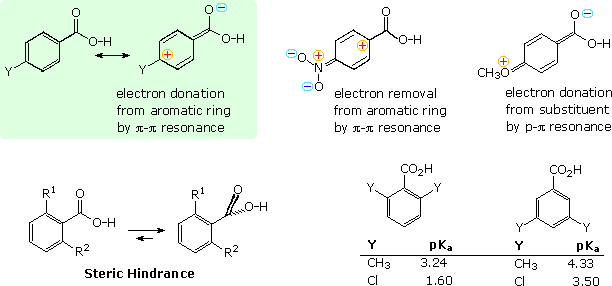
The increased acidity of ortho-substituted benzoic acids is attributed to steric hindrance that forces the carboxyl group to twist out of the plane of the benzene ring. The inductive character of the phenyl group does not change with such twisting, but resonance ( conjugative electron donation ) requires a coplanar relationship. For example, ortho-toluic acid ( R1 = CH3 & R2 = H ) has a pKa of 3.9 compared with 4.2 for benzoic acid itself. If the methyl is changed to a larger tert-butyl group the pKa drops to 3. 53. By sandwiching the carboxyl group between two ortho substituents, it is forced to lie perpendicular to the plane of the aromatic ring, and conjugation is prohibited completely. The dimethyl and dichlorobenzoic acid isomers shown at the lower right in the diagram provide dramatic evidence of this conformational effect, with the bis-ortho (2,6-) isomers representing the exclusive action of the inductive effect.
Steric interference with conjugation may also perturb the acidity of acyclic unsaturated acids. Thus, 2,3-dimethyl-2-butenoic acid has a pKa of 4.41, compared with the 5.12 pKa of 3-methyl-2-butenoic acid.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















