

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Oxygenases
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص754-757
2025-10-25
394
Oxygenases
Key points: Oxygenases catalyse the insertion of one or both O atoms derived from O2 into an organic substrate; monooxygenases catalyse insertion of one O atom while the other O atom is reduced to H2 O; dioxygenases catalyse the incorporation of both O atoms. Oxygenases catalyse the insertion of one or both O atoms of O2 into substrates, whereas with the Fe and Cu containing oxidases both O atoms end up as H2O. Oxygenases are often referred to as hydroxylases when the O atom is inserted into a C-H bond. Most oxygenases contain Fe, the rest contain Cu or flavin, an organic cofactor. There are many variations. Monooxygenases catalyse reactions of the type
R-H+O+2H+ + 2e- → R-O-H+H2O
in which electrons are supplied by an electron donor such as an FeS protein. Monoxyge-nases can also catalyse the epoxidation of alkenes. Dioxygenases catalyse the insertion of both atoms of O2 into substrates, and no additional electron donor is required. Two C H bonds on the same molecule may be oxygenated:
H-R-R-H+O2 → H-O-R-R-O-H
The Fe enzymes are divided into two main classes, haem and non-haem. We discuss the haem enzymes first, the most important type being cytochrome P450. Cytochrome P450 (or just P450) refers to an important and widely distributed group of haem-containing monooxygenases. In eukaryotes, they are localized particularly in mitochondria, and in higher animals they are concentrated in liver tissue. They play an essential role in biosynthesis (for example steroid transformations), such as the production of progesterone. The designation ‘P450’ arises from the intense absorption band that appears at 450 nm when solutions containing the enzyme or even crude tissue extracts are treated with a reducing agent and carbon monoxide, which produces the Fe (II) CO complex. Most P450s are complex membrane-bound enzymes that are difficult to isolate. Much of what we know about them stems from studies carried out with an enzyme P450cam, which is isolated from the bacterium Pseudomonas putida.
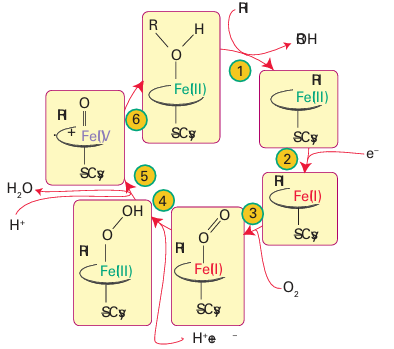
Figure 27.37 The catalytic cycle of cytochrome P450.
This organism uses camphor as its sole source of carbon, and the first stage is oxygenation of the 5-position:
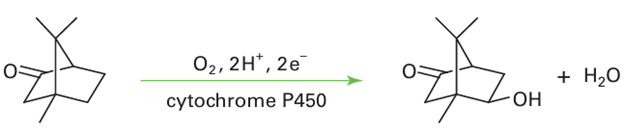
The catalytic cycle has been studied using a combination of kinetic and spectroscopic methods (Fig. 27.37). Starting from the resting enzyme, which is Fe (III), the binding of the substrate in the active site pocket (1) induces release of the coordinated H2 O molecule. This step is detected as a change in spin state from low spin (S=1/2) to high spin (S=5/2) and the re duction potential increases, causing an electron to be transferred (2) from a small [2Fe 2S] containing protein known as putidaredoxin. The five-coordinate Fe (II) that is formed resem bles deoxymyoglobin and binds O2(3). Unlike in myoglobin, addition of a second electron is both thermodynamically and kinetically favourable. The subsequent reactions (4 6) are very fast, but it is thought that an Fe (III) peroxide intermediate is formed that undergoes rapid heterolytic O-O cleavage to produce a species similar to Compound I of peroxidases. In what is known as the oxygen rebound mechanism, the Fe(IV)=O group abstracts an H atom from the substrate and then inserts it back as an OH radical. This process is remark able, as it amounts to the ‘taming’ of an O atom or OH radical by its attachment to Fe.
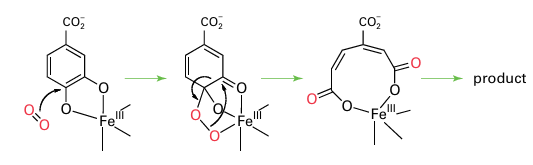
Other P450s are thought to operate by similar mechanisms but differ in the architecture of the active site pocket. That site, unlike the site in peroxidases, is predominantly hydro phobic, with specific polar groups present to orient the organic substrate so the correct R H bond is brought close to the Fe O entity. Non-haem oxygenases are widely distributed and are usually dioxygenases. Most contain a single Fe atom at the active site and are classified according to whether the active species in the protein is Fe (III) or Fe(II). In the Fe (III) enzymes, which are also (historically) known as intradiol oxygenases, the Fe atom functions as a Lewis acid catalyst and activates the organic substrate towards attack by noncoordinating O2:
By contrast, in the Fe (II) enzymes, which are known historically as extradiol oxygenases, the Fe binds O2 directly and activates it to attack the organic substrate:
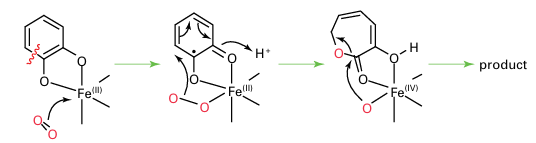
The Fe(III) enzymes are exemplified by protocatechuate 3,4-dioxygenase: the Fe is high spin and tightly coordinated by a set of protein ligands that includes two His-N and two Tyr-O, the latter hard donors being particularly suitable for stabilizing Fe(III) relative to Fe(II). The Fe (III) enzymes are deep red due to an intense tyrosinate-to-Fe (III) charge trans fer transition. The Fe (II) enzymes are exemplified by catechol 2,3-dioxygenase: the Fe is high spin and coordinated within the protein by a set of ligands that includes two His-N and one carboxylate group. The binding is weak, reflecting the low position of Fe (II) in the Irving–Williams series (Section 20.1). This weak binding, together with the difficulty of observing useful spectroscopic features (such as EPR spectra), has made these enzymes much more difficult to study than the Fe (III) enzymes. A particularly important class of Fe (II) oxygenases use a molecule of 2-oxoglutarate as a second substrate:
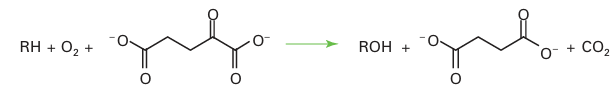
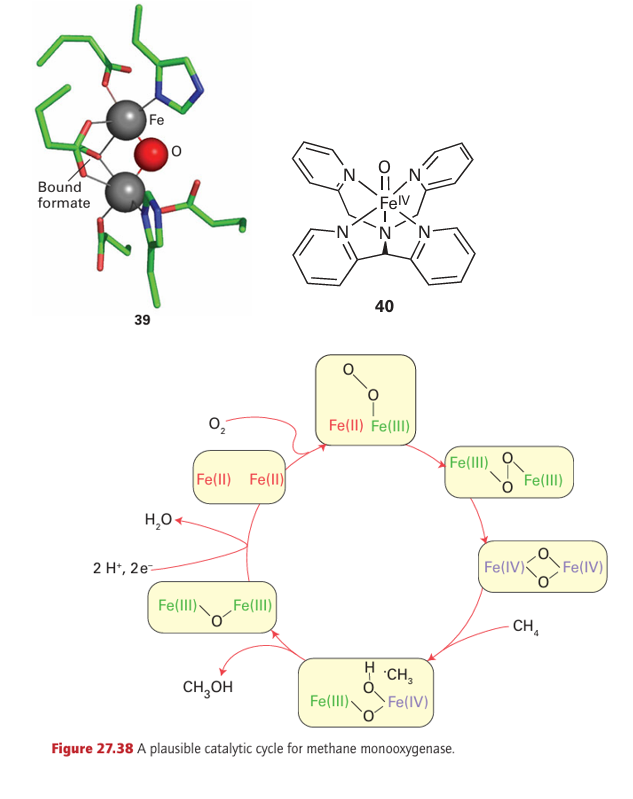
The principle of oxo-glutarate-dependent oxygenases is that the transfer of one O atom of O2 to 2-oxoglutarate (also known as α-ketoglutarate) results in its irreversible decarboxy lation, thus driving insertion of the other O atom into the primary substrate. Examples include enzymes that serve in cell signalling by modifying an amino acid in certain tran scription factors (Section 27.15). Oxygenases play a crucial role in the metabolism of methane, a greenhouse gas. Of all hydrocarbons, methane contains the strongest C–H bonds and is the most difficult to activate. Methane-metabolizing bacteria produce two types of enzyme that catalyse the conversion of methane to methanol (a more useful chemical and fuel) and thus attract much industrial interest. One is a membrane-bound enzyme that contains Cu atoms. This enzyme, known as ‘particulate’ methane monooxygenase (p-mmo), is expressed when high levels of Cu are available. The other enzyme, soluble methane monooxygenase (s-mmo), contains a dinuclear Fe active site (39) that is related to haemerythrin (21) and acid phosphatase (33). The mechanisms are not established but Fig. 27.38 shows a plausible catalytic cycle for s-mmo. The intermediate Fe (IV) species that is proposed differs from those we have encountered up to now, as the O2-derived oxido ligands are bridging rather than terminal. Despite the importance of Fe (IV) as an enzyme intermediate, small Fe (IV) complexes that could provide important models for understanding the enzymes have been elusive. The easiest to prepare are haem analogues in which Fe is equatorially ligated by porphyrin and which can be formed by reacting the Fe (II) or Fe (III) forms with a peroxo acid. Small models of non-haem Fe (IV) species have now been prepared. The mononuclear complex (40) containing the pentadentate pentaaza ligand N, N-bis(2-pyridylmethyl)-N bis(2-pyridyl) methylamine is formed by treating the Fe (II) complex with the oxo-transfer agent iodosylbenzene. It is fairly stable at room temperature and has been structurally characterized by X-ray diffraction. A powerful oxidizing agent, it can also be generated in acetonitrile solution by bulk electrolysis of the Fe (II) complex in the presence of water, and the standard potential for Fe (IV)/Fe (III) is estimated to be 0.9 V relative to the ferro cinium/ferrocene couple. Complex (40) and similar species are paramagnetic (S=1) and show characteristic absorption bands in the near-infrared. The Fe–O bond length in (40) is 164 pm, which is fully consistent with a multiple bond in which the O atom is acting as a π donor. Complex (40) is able to oxygenate C–H bonds in a variety of hydrocarbons,
including cyclohexane. The bis(µ-oxo)Fe(IV) complex (41) has been proposed as a structural analogue of the reactive intermediate formed in s-mmo. Tyrosinase and catechol oxidase, two enzymes responsible for producing melanin-type pigments, each contain a strongly coupled dinuclear Cu centre that coordinates O2 in a manner similar to haemocyanin. However, unlike in haemocyanin, the ligands *-to-Cu charge transfer is enhanced sufficiently to activate the coordinated O2 for electrophilic attack at a phenolic ring of the substrate. The structure of the active site of catechol oxidase complexed with the inhibitor phenolthiourea (42) shows how the phenol ring of the substrate can be oriented in close proximity to a bridging O2. Copper enzymes are also responsible for the production of important neurotransmitters and hormones, such as dopamine and noradrenaline. These enzymes contain two Cu atoms that are well separated in space and uncoupled magnetically.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















